Hymeglusin, Fusarilactone A
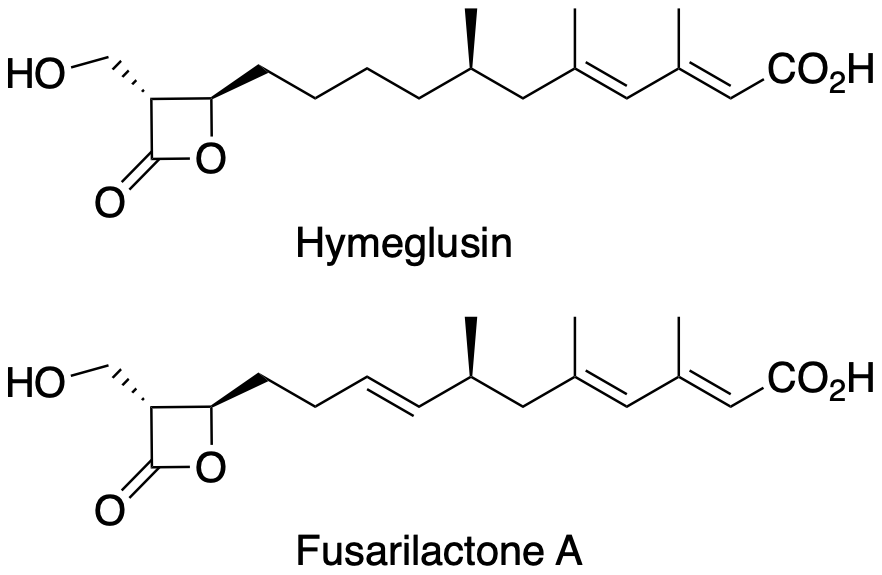
Total Syntheses and Chemical Biology Studies of Hymeglusin and Fusarilactone A, Novel Circumventors of β-Lactam Drug Resistance in Methicillin-Resistant Staphylococcus aureus
M. Kanaida※, A. Kimishima※, S. Eguchi, M. Iwatsuki, Y. Watanebe, M. Honsho, T. Hirose, Y. Noguchi, K. Nonaka, G. Sennari, H. Matsui, C. Kaito, H. Hanaki, Y. Asami※※, T. Sunazuka※※.
※These authors contributed equally to this work. ※※ Corresponding authors.
ChemMedChem, 2021, 16, 2106.
Diatretol, Lepistamide A, B, C
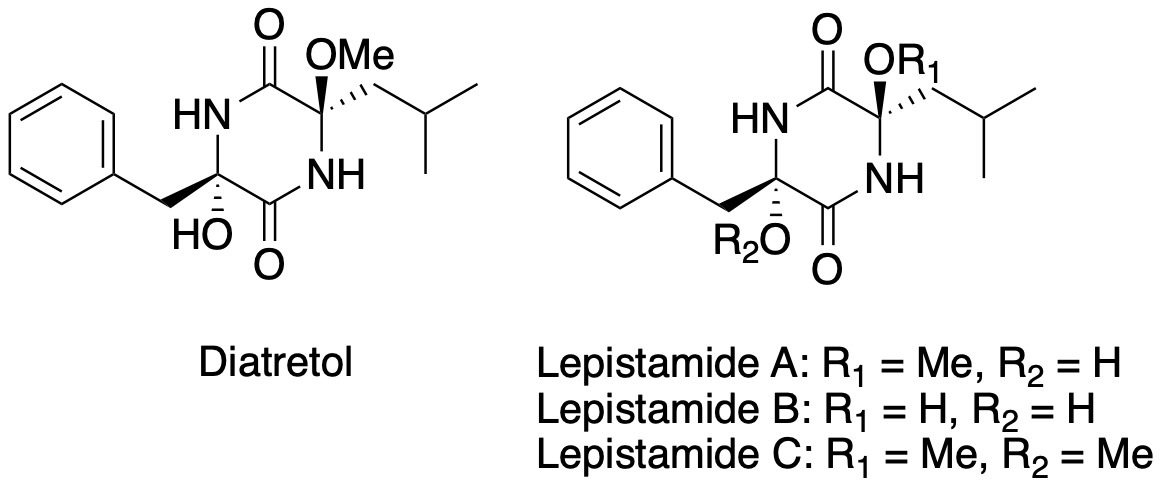
Unified enantioselective total synthesis of 3,6-dioxygenated diketopiperazine natural products, diatretol and lepistamides A, B and C
S. Takahashi, A. Kimishima, T. Hirose, T. Yamada, A. Sugawara, Y. Noguchi, M. Iwatsuki, R. Hokari, A. Ishiyama, Y. Kobayashi, T. Sunazuka.
Tetrahedron Letters, 2021, 67, 152895.
Kozupeptin A
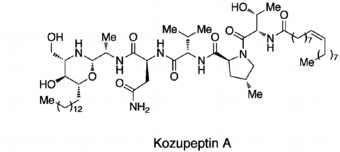
Kozupeptins, Antimalarial Agents Produced by Paracamarosporium Species: Isolation, Structural Elucidation, Total Synthesis, and Bioactivity.
Hayashi Y, Fukasawa W, Hirose T, Iwatsuki M, Hokari R, Ishiyama A, Kanaida M, Nonaka K, Takè A, Otoguro K, Omura S, and Sunazuka T.
Org. Lett., 2019, 21, 2180.
(+)-Mangromicin A
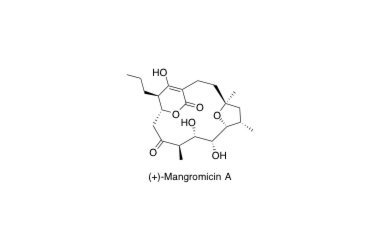
Total Synthesis and Determination of the Absolute Configuration of Naturally Occurring Mangromicin A, with Potent Antitrypanosomal Activity
Hirokazu Takada, Takeshi Yamada, Tomoyasu Hirose, Takuma Ishihara, Takuji Nakashima, Yo̅ko Takahashi, Satoshi O̅mura and Toshiaki Sunazukma
Org. Lett. 2017, 19, 230-233.
(±)-Naphthacemycin A9
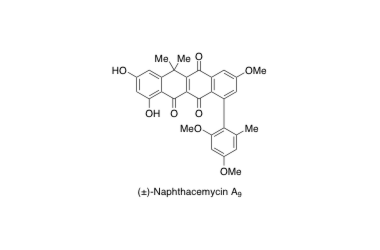
Total synthesis of (±)-naphthacemycin A9, possessing both antibacterial activity against methicillin-resistant Staphylococcus aureus and circumventing effect of β-lactam resistance
Tomoyasu Hirose, Yasuhiro Kojima, Hidehito Matsui, Hideaki Hanaki, Masato Iwatsuki, Kazuro Shiomi, Satoshi Ōmura and Toshiaki Sunazuka
J. Antibiot. 2016, advance online publication.
DOI: 10.1038/ja.2016.141
Neoxaline, Meleagrin A, Oxaline
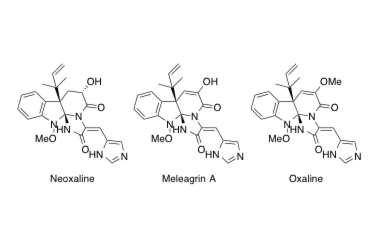
Asymmetric Total Synthesis of Indole Alkaloids Containing An Indoline Spiroaminal Framework.
Yamada, T.; Ideguchi-Matsushita, T.; Hirose, T.; Shirahata, T.; Hokari, R.; Ishiyama, A.; Iwatsuki, M.; Sugawara, A.; Kobayashi, Y.; Otoguro, K.; Ōmura S. and Sunazuka T.
Chem. E. J. 2015, 21, 11855-11864.
Jietacin A, B, C, D

Jietacins with potent nematocidal activity; efficient isolation of novel analogues and divergent total synthesis of jietacin A, B, C, and D
Sugawara, A.; Kubo, M.; Nakashima, T.; Hirose, T.; Tsunoda, N.; Yahagi, K.; Asami, Y.; Yamada, T.; Shiomi, K.; Takahashi, Y.; Ōmura, S. and Sunazuka, T.
Tetrahedron 2015, 71, 2149-2157.
Puberulic acid
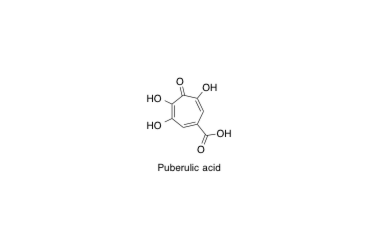
A concise total synthesis of puberulic acid, a potent antimalarial agent
Sennari, G.; Hirose, T.; Iwatsuki, M.; Ōmura, S. and Sunazuka, T.
Chem. Commun. 2014, 50, 8715-8718.
Neoxaline
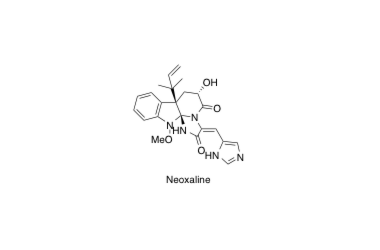
Asymmetric Total Synthesis of Neoxaline
Ideguchi, T.; Yamada, T.; Shirahata, T.; Hirose, T.; Sugawara, A.; Kobayashi, Y.; Omura, S. and Sunazuka, T.
J. Am. Chem. Soc. 2013, 135, 12568-12571.
(+)-16-Hydroxy-16,22-dihydroapparicine
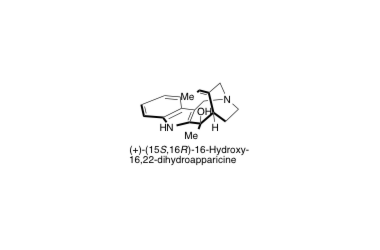
Structure Determination and Total Synthesis of (+)-16-Hydroxy-16,22-dihydroapparicine
Hirose, T.; Noguchi, Y.; Furuya, Y.; Ishiyama, A.; Iwatsuki, M.; Otoguro, K.; Ōmura, S. and Sunazuka, T.
Chem. Eur. J. 2013, 19, 10741-10750.
(±)-16-Hydroxy-16,22-dihydroapparicine
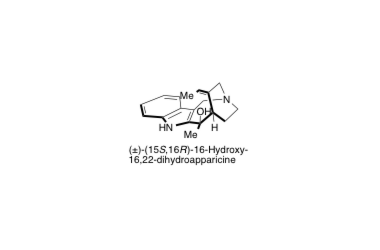
The first total synthesis and reassignment of the relative stereochemistry of 16-hydroxy-16,22-dihydroapparicine
Noguchi, Y.; Hirose, T.; Furuya, Y.; Ishiyama, A.; Otoguro, K.; Ōmura, S. and Sunazuka, T.
Tetrahedron Lett. 2012, 53, 1802-1807.
Argifin
Solution-phase total synthesis of the hydrophilic natural product argifin using 3,4,5-tris(octadecyloxy)benzyl tag.
Hirose, T.; Kasai, T.; Akimoto, T.; Endo, A.; Sugawara, A.; Nagasawa, K.; Shiomi, K.; Ōmura, S. and Sunazuka, T.
Tetrahedron 2011, 67, 6633-6643.
(Review; Proc. Jpn. Acad., Ser. B, 2010, 86, 85-102.)
Guadinomine B2, C, Guadinomic acid
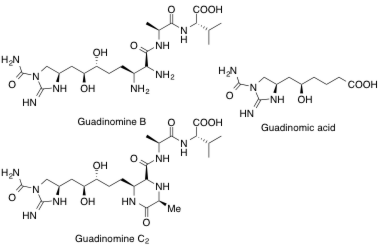
Isolation, total synthesis and determination of the absolute configuration of Guadinomines; potent inhibitors of a bacterial type III secretion system
Hirose, T.; Iwatsuki, M.; Ōmura, S. and Sunazuka, T.
J. Synth. Org. Chem. Jpn. 2011, 69, 775-788.
Malformin C
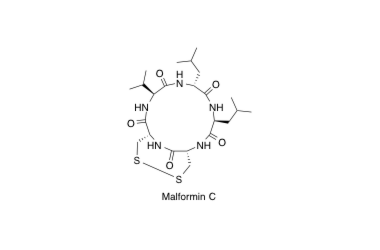
Solid-phase synthesis and biological activity of malformin C and its derivatives
Kojima, Y.; Sunazuka, T.; Nagai, K.; Hirose, T.; Namatame, M.; Ishiyama, A.; Otoguro, K. and Ōmura, S.
J. Antibiot. 2009, 62, 681-686.
Argadin
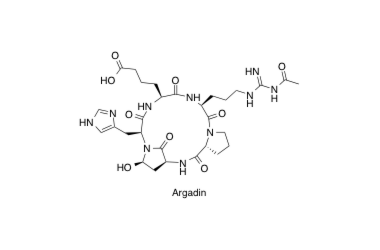
Solid phase total synthesis of chitinase inhibitor Argadin using a supported acetal resin
Hirose, T.; Sunazuka, T.; Sugawara, A.; Noguchi, Y.; Tanaka, T.; Iguchi, K.; Yamamoto, T.; Gouda, H.; Shiomi, K. and Ōmura, S.
J. Antibiot. 2009, 62, 495-500.
(Review; Proc. Jpn. Acad., Ser. B, 2010, 86, 85-102.)
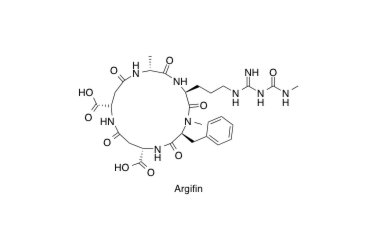
Argifin
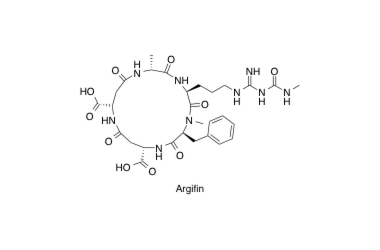
Argifin; Efficient Solid Phase Total Synthesis and Unexpected Discovery of potent Acyclic peptides
Sunazuka, T.; Sugawara, A.; Iguchi, K.; Hirose, T.; Nagai, K.; Noguchi, Y.; Saito, Y.; Gouda, H.; Yamamoto, T.; Ui, H.; Shiomi, K. and Ōmura, S.
Bioorg. Med. Chem. 2009, 17, 2751-2758.
(Review; Proc. Jpn. Acad., Ser. B, 2010, 86, 85-102.)
Bottromycin A2
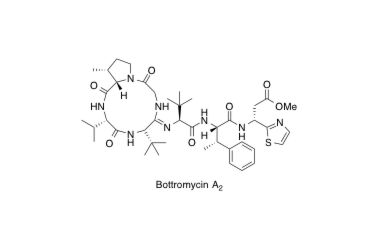
Structure Determination and Total Synthesis of Bottromycin A2: A Potent Antibiotic against MRSA and VRE
Shimamura, H.; Gouda, H.; Nagai, K.; Hirose, T.; Ichioka, M.; Furuya, Y.; Kobayashi, Y.; Hirono, S.; Sunazuka, T. and Ōmura, S.
Angew. Chem. Int. Ed. 2009, 48, 914-917.
Guadinomine B, C2
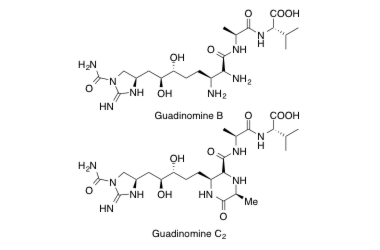
Total Synthesis and Determination of the Absolute Configuration of Guadinomines B and C2
Hirose, T.; Sunazuka, T.; Tsuchiya, S.; Tanaka, T.; Kojima, Y.; Iwatsuki, M. and Ōmura, S.
Chem. Eur. J. 2008, 14, 8220-8238.
Malformin C
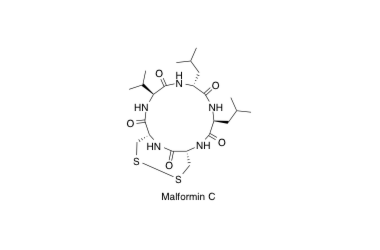
Total synthesis of malformin C, an inhibitor of bleomycin-induced G2 arrest
Kojima, Y.; Sunazuka, T.; Nagai, K.; Julfakyan, K.; Fukuda, T.; Tomoda, H. and Ōmura, S.
J. Antibiot. 2008, 61, 297-302.
Tensyuic acid B, C, E
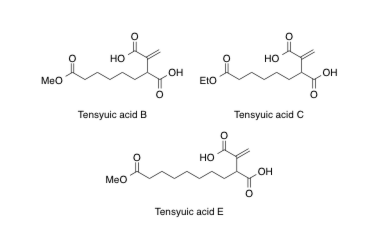
Synthesis and biological properties of tensyuic acids B, C, and E, and investigation of the optical purity of natural tensyuic acid B.
Matsumaru, T.; Sunazuka, T.; Hirose, T.; Ishiyama, A.; Namatame, M.; Fukuda, T.; Tomoda, H.; Otoguro, K. and Ōmura, S.
Tetrahedron 2008, 64, 7369-7377.
Verticipyrone
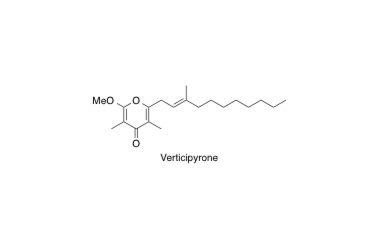
Total Synthesis and Biological Evaluation of Verticipyrone and Analogues.
Shimamura, H.; Sunazuka, T.; Izuhara, T.; Hirose, T.; Shiomi, K. and Ōmura, S.
Org. Lett. 2007, 9, 65-67.
K01-0509 B
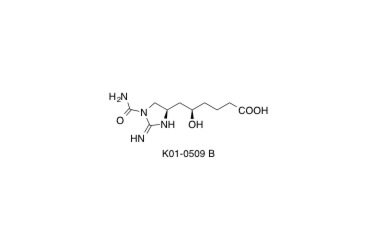
Asymmetric Total Synthesis of (+)-K01-0509 B: Determination of Absolute Configuration.
Tsuchiya, S.; Sunazuka, T.; Hirose, T.; Mori, R.; Tanaka, T.; Iwatsuki, M. and Ōmura, S.
Org. Lett. 2006, 8, 5577-5580.
Verticilide
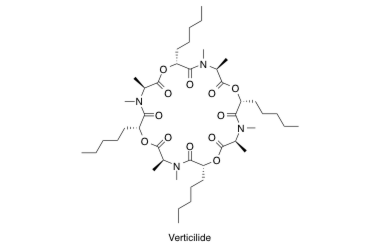
Verticilide: Elucidation of Absolute Configuration and Total Synthesis.
Monma, S.; Sunazuka, T.; Nagai, K.; Arai, T.; Shiomi, K.; Matsui, R. and Ōmura, S.
Org. Lett. 2006, 8, 5601-5604.
Madindoline A, B

Synthetic applications of a three-component Mannich reaction. Total synthesis of IL-6 inhibitor (+)-madindoline A and B.
Hirose, T.; Sunazuka, T.; Yamamoto, D.; Kaji, E. and Ōmura, S.
Tetrahedron Lett. 2006, 47, 6761-6764.
Beauveriolide
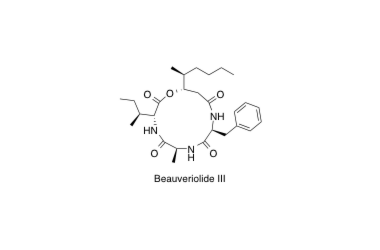
Synthesis and Biological Evaluation of a Beauveriolide Analogue Library.
Nagai, K.; Doi, T.; Sekiguchi, T.; Namatame, I.; Sunazuka, T.; Tomoda, H.; Ōmura, S. and Takahashi, T.
J. Comb. Chem. 2006, 8, 103-109.
Madindoline A, B
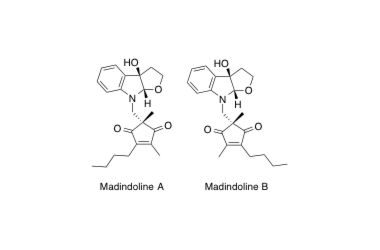
Determination of the absolute stereochemistry and asymmetric total synthesis of madindolines A and B: a practical improvement to a second-generation approach from the first-generation.
Hirose, T.; Sunazuka, T.; Yamamoto, D.; Kojima, N.; Shirahata, T.; Harigaya, Y.; Kuwajima, I. and Ōmura, S.
Tetrahedron 2005, 61, 6015-6039.
Macrosphelide A, E
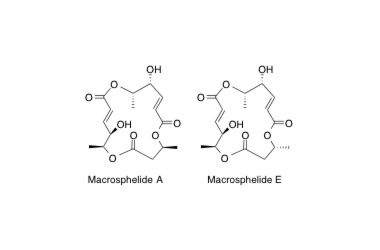
Absolute stereochemistries and total synthesis of (+)/(-)-macrosphelides, potent, orally bioavailable inhibitors of cell-cell adhesion.
Sunazuka, T.; Hirose, T.; Chikaraishi, N.; Harigaya, Y.; Hayashi, M.; Komiyama, K.; Sprengeler, P. A.; Smith, A. B. and Ōmura, S.
Tetrahedron 2005, 61, 3789-3803.
(Acc. Chem. Res. 2008, 41, 302–314.)
Physovenine
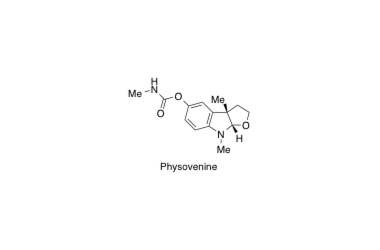
Total synthesis of (–)-physovenine from (–)-3a-hydroxyfuroindoline.
Sunazuka, T.; Yoshida, K.; Kojima, N.; Shirahata, T.; Hirose, T.; Handa, M.; Yamamoto, D.; Harigaya, Y.; Kuwajima, I. and Ōmura, S.
Tetrahedron Lett. 2005, 46, 1459-1461.
Arisugacin A, B
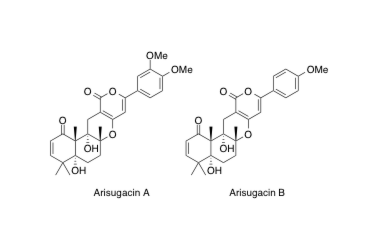
Absolute stereochemistries and total synthesis of (+)-arisugacins A and B, potent, orally bioactive and selective inhibitors of acetylcholinesterase.
Sunazuka, T.; Handa, M.; Nagai, K.; Shirahata, T.; Harigaya, Y.; Otoguro, K.; Kuwajima, I. and Ōmura, S.
Tetrahedron 2004, 60, 7845-7859.
Arisugacin F, G
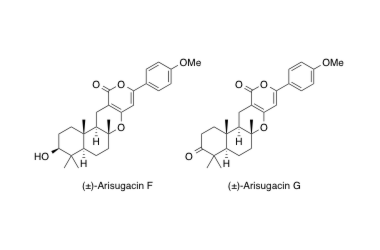
Total syntheses of the AChE inhibitors (±)-arisugacins F and G.
Handa, M.; Sunazuka, T.; Sugawara, A.; Harigaya, Y.; Otoguro, K. and Ōmura, S.
J. Antibiot. 2003, 56, 730-733.
Pinellic acid
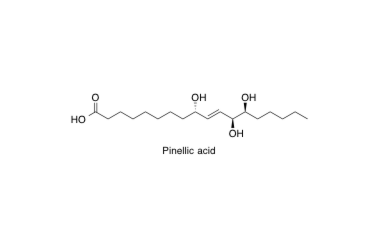
Total synthesis and adjuvant activity of all stereoisomers of pinellic acid.
Shirahata, T.; Sunazuka, T.; Yoshida, K.; Yamamoto, D.; Harigaya, Y.; Nagai, T.; Kiyohara, H.; Yamada, H.; Kuwajima, I. and Ōmura, S.
Bioorg. Med. Chem. Lett. 2003, 13, 937-941.
Pinellic acid

Total synthesis of pinellic acid, a potent oral adjuvant for nasal influenza vaccine. Determination of the relative and absolute configuration.
Sunazuka, T.; Shirahata, T.; Yoshida, K.; Yamamoto, D.; Harigaya, Y.; Nagai, T.; Kiyohara, H.; Yamada, H.; Kuwajima, I. and Ōmura, S.
Tetrahedron Lett. 2002, 43, 1265-1268.
Madindoline A, B
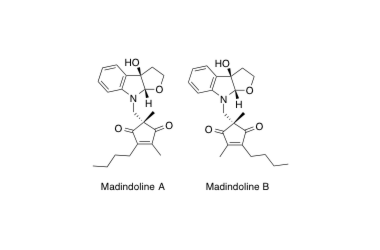
Short Total Synthesis of (+)-Madindolines A and B.
Hirose, T.; Sunazuka, T.; Shirahata, T.; Yamamoto, D.; Harigaya, Y.; Kuwajima, I. and Ōmura, S.
Org. Lett. 2002, 4, 501-503.
Arisugacin A
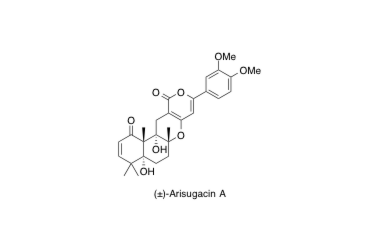
The First Total Synthesis of (±)-Arisugacin A, a Potent, Orally Bioavailable Inhibitor of Acetylcholinesterase.
Sunazuka, T.; Handa, M.; Nagai, K.; Shirahata, T.; Harigaya, Y.; Otoguro, K.; Kuwajima, I. and Ōmura, S.
Org. Lett. 2002, 4, 367-369.
(Acc. Chem. Res. 2008, 41, 302–314.)
(Review; Chem. Rev. 2005, 105, 4559–4580.)
Kurasoin A, B
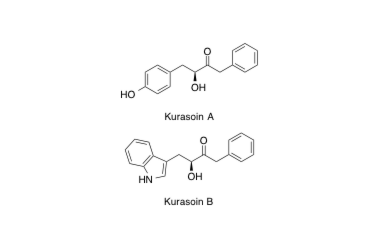
Total Syntheses of Kurasoins A and B, Novel Protein Farnesyltransferase Inhibitors, and Absolute Structures of Kurasoins A and B.
Hirose, T.; Sunazuka, T.; Zhi-Ming, T.; Handa, M.; Uchida, R.; Shiomi, K.; Harigaya, Y. and Ōmura S.
Heterocycles 2000, 53, 777-784.
Madindoline A, B
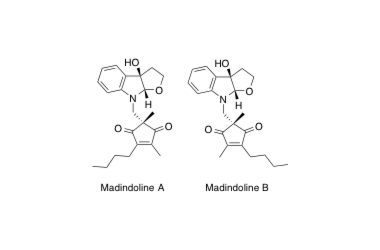
Total Synthesis of (+)-Madindoline A and (−)-Madindoline B, Potent, Selective Inhibitors of Interleukin 6. Determination of the Relative and Absolute Configurations.
Sunazuka, T.; Hirose, T.; Shirahata, T.; Harigaya, Y.; Hayashi, M.; Komiyama, K. and Ōmura, S.
J. Am. Chem. Soc. 2000, 122, 2122-2123.
Macrosphelide A, B
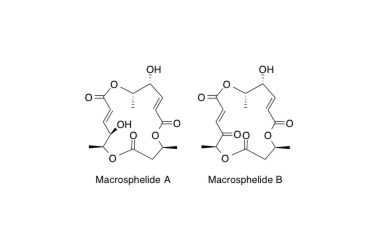
Relative and Absolute Stereochemistries and Total Synthesis of (+)-Macrosphelides A and B, Potent, Orally Bioavailable Inhibitors of Cell−Cell Adhesion.
Sunazuka T.; Hirose, T.; Harigaya, Y.; Takamatsu, S.; Hayashi, M.; Komiyama, K. and Ōmura, S.
J. Am. Chem. Soc. 1997, 119, 10247-10248.
Kurasoin A, B
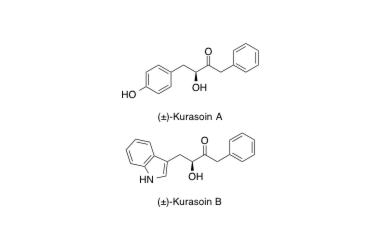
Kurasoins A and B, New Protein Earnesyltransferase Inhibitors Produced by Paedlomyces sp. FO-3684.
Uchida, R.; Shiomi, K.; Sunazuka, T.; Inokoshi, J.; Nishizawa, A.; Hirose, T.; Tanaka, H.; Iwai, Y. and Ōmura S.
J. Antibiot. 1996, 49, 886-889.
Pyripyropene A
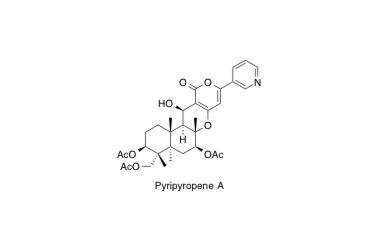
Total Synthesis of (+)-Pyripyropene A, a Potent, Orally Bioavailable Inhibitor of Acyl-CoA:Cholesterol Acyltransferase.
Nagamitsu, T.; Sunazuka, T.; Obata, R.; Tomoda, H.; Tanaka, H.; Harigaya, Y.; Ōmura, S. and Amos B. Smith III
J. Org. Chem. 1995, 60, 8126-8127.
(Acc. Chem. Res. 2008, 41, 302–314.)
(Review; Chem. Rev. 2005, 105, 4559–4580.)
Lactacystin
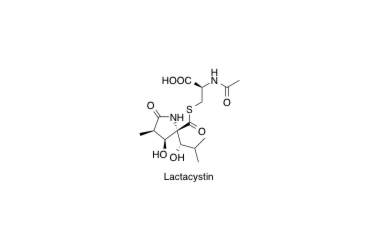
Total synthesis of (+)-lactacystin, the first non-protein neurotrophic factor.
Sunazuka, T.; Nagamitsu, T.; Matsuzaki, K.; Tanaka, H.; Ōmura, S. and Amos B. Smith III
J. Am. Chem. Soc. 1993, 115, 5302-5302.
(Acc. Chem. Res. 2008, 41, 302–314.)
Diolmycin A1
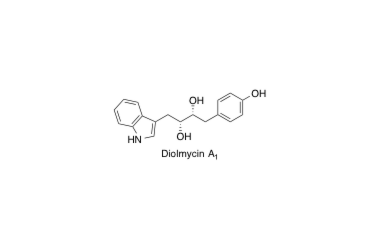
Asymmetric synthesis of the anticoccidial antibiotic diolmycin A1. Determination of absolute stereostry.
Snazuka, T.; Tabata, N.; Nagamitsu, T.; Tomoda, H.; Ōmura S. and Amos B. Smith III
Tetrahedron Lett. 1993, 34, 6659-6660.