各年をクリックすると、その年の論文の一覧をご覧いただけます。
2026
In situ クリックケミストリーを利用した酵素阻害剤の探索
2025
Late-Stage Native Peptide Modification Approach to the Total Synthesis of Koshidacins A and B

Nakahara, H.†; Okano, T.†; Sennari, G.*; Iwatsuki, M.; Sunazuka, T.; Hirose, T.*
JACS Au, 2025, 5, 5932–5938.
A 28-day subacute toxicity study of puberulic acid in Crl:CD(SD) rats.
Matsushita, K.; Tsuji, G.; Akane, H.; Ishii, Y.; Takasu, S.; Ogawa, K.; Ito, T.; Yokoo, H.; Sennari, G.; Iwatsuki, M.; Hirose, T.; Hanaki, H.; Demizu, Y.; Hirabayashi, Y.; Saito, Y.; Honma, M.; Toyoda, T.*
J. Toxicol. Pathol. 2025, 38, 223–236.
Ufisonitriles A and B, Antimalarial Isonitriles with Mitochondrial Function Inhibitory Activity Produced by Amycolatopsis sp. OK19-0009.
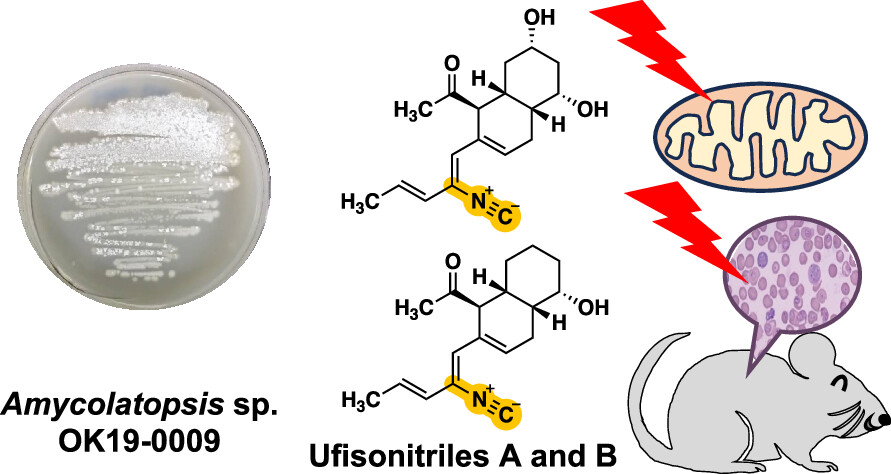
Arakawa, E.; Watanabe, Y.; Tsutsumi, H.; Ikeda, A.; Hirose, T.; Sato, N.; Chinen, T.; Take, A.; Kanto, H.; Inahashi, T.; Teruya, T.; Sunazuka, T.; Hanaki, H.; Usui, T.; Hokari, R.; Ishiyama, A.*; Asami, Y.*; Iwatsuki, M.*
J. Nat. Prod. 2025, 88, 2472–2480.
Harnessing the Biosynthetic Diversity of Actinomycetes: Discovery of Unique Natural Products Through Comparative Genomic and Metabolic Analysis.
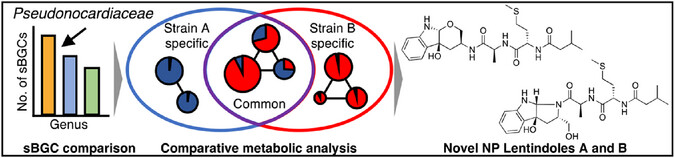
Kikuchi, Y.; Tsutsumi, H.; Watanabe, Y.; Nakahara, H.; Ito, S.; Noguchi, Y.; Awano, Y.; Kasuga, M.; Iwatsuki, M.; Hirose, T.; Sunazuka, T.; Inahashi, Y.*
Chem. A. Eur. J. 2025, 31, e01912.
Discovery of Structurally Diverse γ-Pyrone-Type Diterpenoids Produced by the Fungus Mariannaea sp. FKR-1011 from Zamami Island.
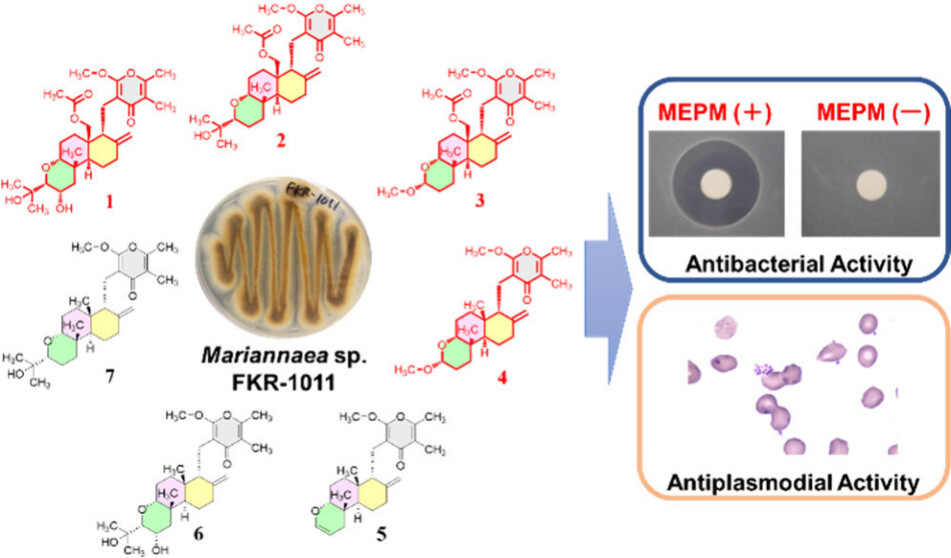
Tani, K.; Wakatsuki, M.; Wada, M.; Nonaka, K.; Kikuchi, Y.; Kimura, S.; Watanabe, Y.; Hokari, R.; Hirose, T.; Honsho, M.; Asami, Y.; Tsutsumi, H.; Inahashi, Y.; Iwatsuki, M.; Matsui, H.; Sunazuka, T.; Hanaki, H.; Teruya, T.; Ishii, T.*
J. Nat. Prod. 2025, 88, 1871–1878.
Macrocyclic Skeletal Modification Approach to the Anti-Trypanosomal Macrolides, Actinoallolides.
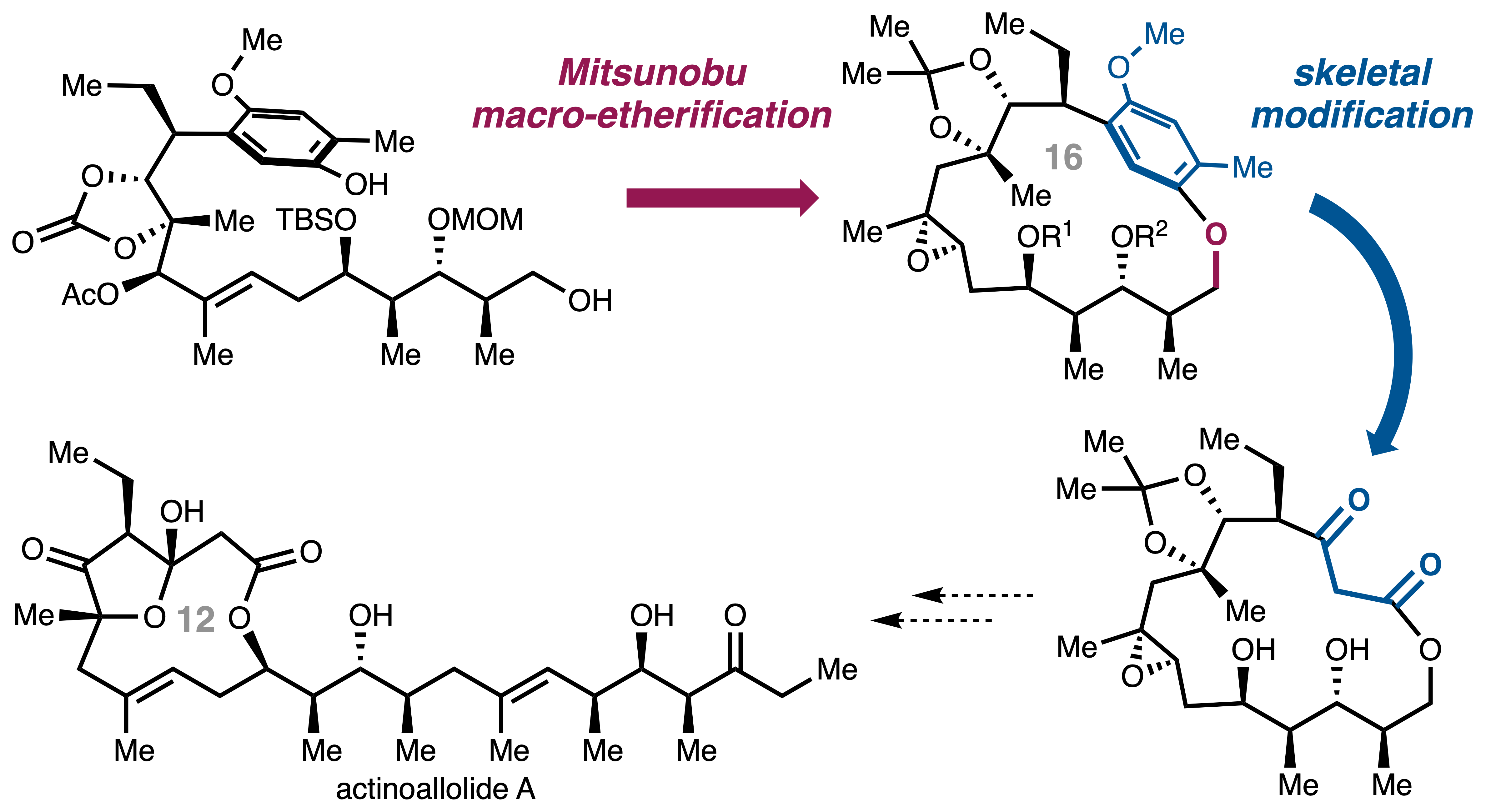
Sennari, G.; Akito Watanabe; Takanori Ōno; Jun Oshita; Yoshihiko Noguchi; Hirose, T.*; Sunazuka, T.*
Chem. Commun. 2025, 61, 10367–10370.
Total Synthesis of Puberulic Acid and Related Congeners Using a Carbohydrate Framework Source.
糖をC–O骨格源として用いたプベルル酸と類縁天然物の全合成
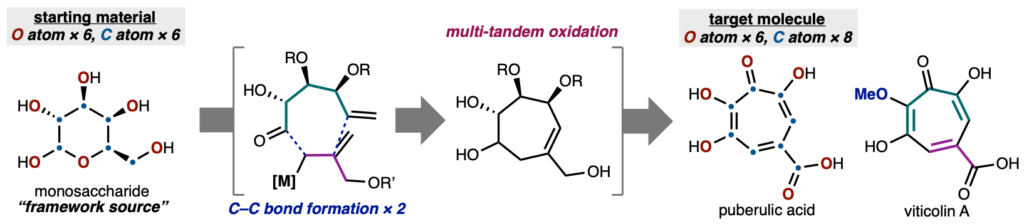
Sennari, G.; Hirose, T.; Sunazuka, T.*
J. Synth. Org. Chem., Jpn. 2025, 83, 293–304.
Isolation, total synthesis and structure determination of antifungal macrocyclic depsipeptide, tetraselide
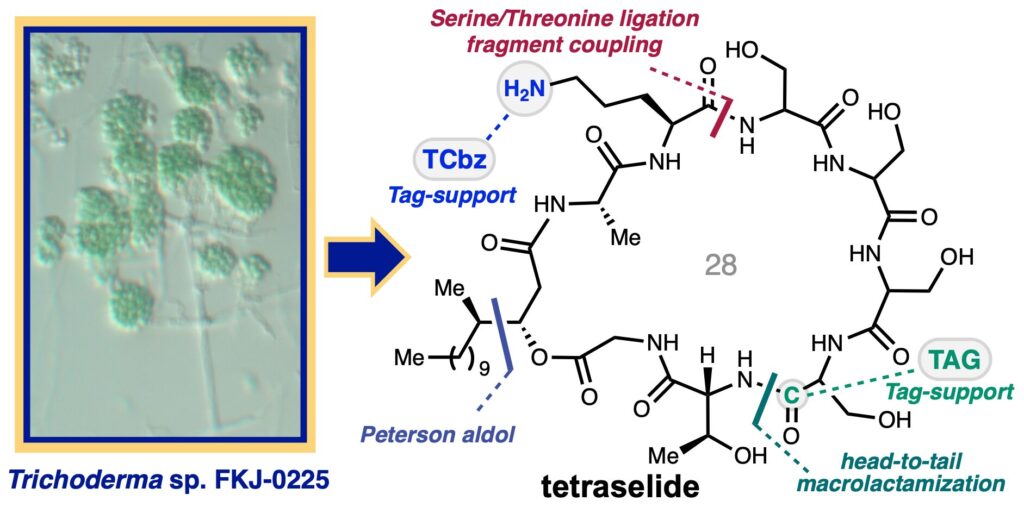
Nakahara, H.; Sennari, G.; Azami, H.; Tsutsumi, H.; Watanabe, Y.; Noguchi, Y.; Inahashi, Y.; Iwatsuki, M.; Hirose, T.*; Sunazuka, T.*
Chem. Sci. 2025, 16, 6060–6069.
Palladium-Catalyzed Reductive and Redox-Neutral Cyclization Approach to the Southern Core of Avermectins
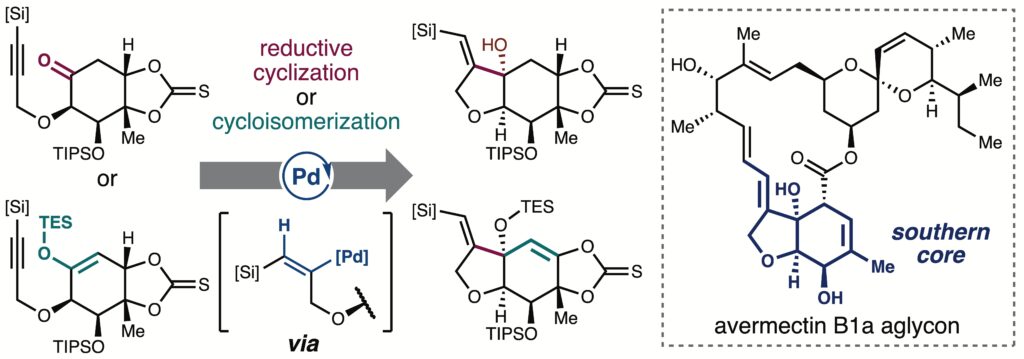
Sennari, G.; Sato, S.; Kimishima, A.; Hirose, T.; Sunazuka T.*
Org. Lett. 2025, 27, 328–333.
2024
In vitro and in vivo activities of KSP-1007, a broadspectrum inhibitor of serine- and metallo-β-lactamases, in combination with meropenem against carbapenem-resistant Gram-negative bacteria.
Takemoto, K.; Nakayama, R.; Fujimoto, Koichi; Suzuki, Y.; Takarabe, Y.; Honsho, M.; Kitahara, S.; Noguchi, Y.; Matsui, H.; Hirose, T.; Asami, Y.; Hidaka, J.; Sunazuka, T.; Hanaki, H.*
Antimicrob. Agents Chemother. 2024, 68, 1–20.
A novel 12-membered ring non-antibiotic macrolide EM982 attenuates cytokine production by inhibiting IKKβ and IκBα phosphorylation.
Saito, R.; Domon, H.; Hiyoshi, T.; Hirayama, S.; Maekawa, T.; Takenaka, S.; Noiri, Y.; Ikeda, A.; Hirose, T.; Sunazuka, T.; Terao, Y.*
J. Biolog. Chem. 2024, 300, 107384.
C–H functionalization of camphor through emerging approaches
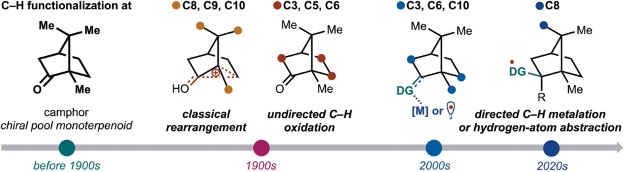
Sennari, G.; Yamagishi, H.; Sarpong R.*
Chem. Lett. 2024, 53, upae204.
千成 恒先生のSarpong研での研究成果です。
Late-stage benzenoid-to-troponoid skeletal modification of the cephalotanes exemplified by the total synthesis of harringtonolide
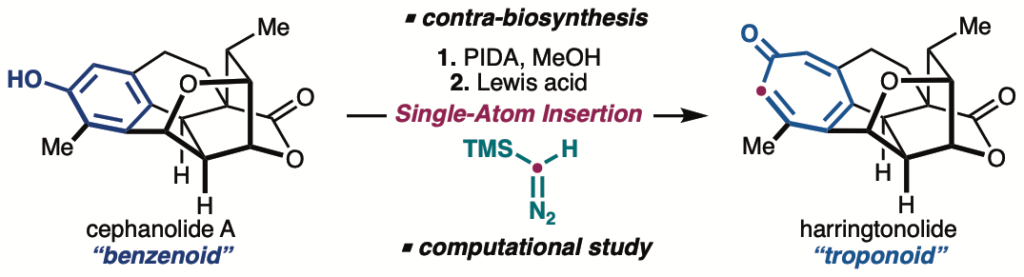
Wiesler, S.†; Sennari, G.†; Popescu, M. V.; Gardner, K. E.; Aida, K.; Paton, R. S.; Sarpong, R.*
Nat. Commun. 2024, 15, 4125.
千成 恒先生のSarpong研での研究成果です。
Remote C–H Amination and Alkylation of Camphor at C8 through Hydrogen-Atom Abstraction
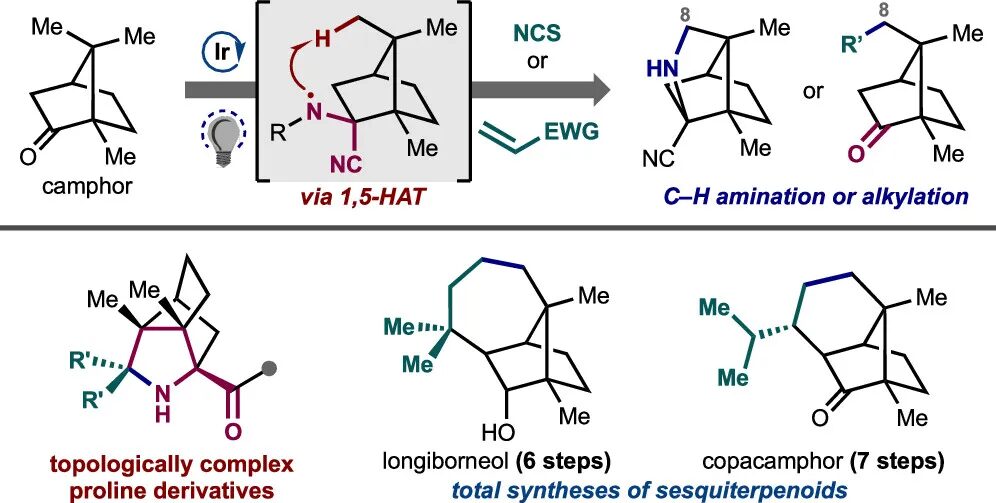
Sennari, G.*; Yamagishi, H.; Sarpong, R.*
J. Am. Chem. Soc. 2024, 146, 7850–7857.
千成 恒先生のSarpong研での研究成果です。
A new analog of dihydroxybenzoic acid from Saccharopolyspora sp. KR21-0001
R. Janthanom, Y. Kikuchi, H. Kanto, T. Hirose, T. Ishii, A. Thamchaipenet, Y. Inahashi.
Belistein J. Org. Chem., 2024, 20, 497-503.
A Novel Macrolide–Del-1 Axis to Regenerate Bone in Old Age
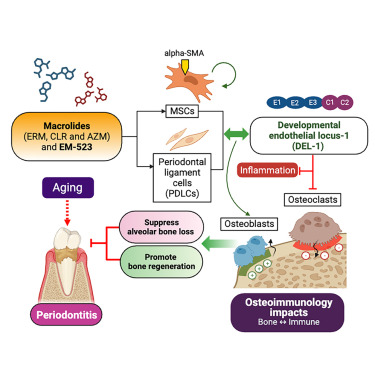
K. Sirisereephap, H. Tamura, J.-H. Lim, M. D. C. Surboyo, T. Isono, T. Hiyoshi, A. L. Rosenkranz, Y. Sato-Yamada, H. Domon, A. Ikeda, T. Hirose, T. Sunazuka, N. Yoshiba, H. Okada, Y. Terao, T. Maeda, K. Tabeta, T. Chavakis, G. Hajishengallis, T. Maekawa.
iScience, 2024, 27, 108798.
Virgaricins C and D, new pramanicin analogs produced by Apiospora sp. FKI-8058.
S. Kimura, Y. Watanabe, Y. Mikasa, R. Miyano, T. Tokiwa, K. Nonaka, T. Nakashima, Y. Noguchi, T. Hirose, T. Sunazuka, R. Hokari, A. Ishiyama, M. Iwatsuki.
J. Antibiot., 2024, 77, 206-213.
The potentiation activity of β-lactam by phomoidrides and oxasetin against methicillin-resistant Staphylococcus aureus
M. Honsho, A. Kimishima, A. Ikeda, M. Iwatsuki, K. Nonaka, H. Matsui, H. Hanaki, Y. Asami, T. Sunazuka.
J. Antibiot., 2024, 77, 185-188.
Antifungal profile against Candida auris clinical isolates of tyroscherin and its new analog produced by the deep-sea-derived fungal strain Scedosporium apiospermum FKJ-0499
H. Azami, Y. Watanabe, K. Sakai, H. Nakahara, T. Tokiwa, K. Nonaka, Y. Noguchi, Y. Nagano, T. Hirose, T. Sunazuka, H. Matsui, N. Arima, K. Abe, H. Hanaki, H. Kojima, M. Iwatsuki.
J. Antibiot., 2024, 77, 156-162.
A Potent PDK4 Inhibitor for Treatment of Heart Failure with Reduced Ejection Fraction
K. Aizawa, A. Ikeda, S. Tomida, K. Hino, Y. Sugita, T. Hirose, T. Sunazuka, H. Kido, S. Yokoyama, R. Nagai .
Cells, 2024, 13, 87.
2023
Use of Emerging C–H Functionalization Methods to Implement Strategies for the Divergent Total Syntheses of Bridged Polycyclic Natural Products
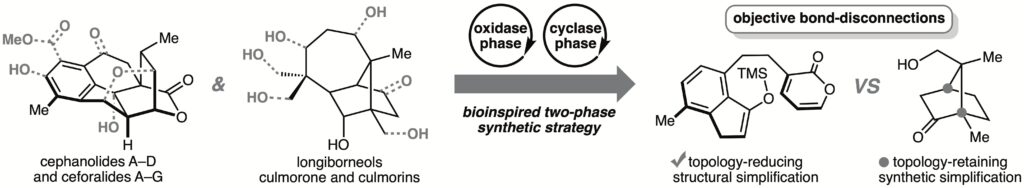
Sennari, G.*; Sarpong, R.*
J. Synth. Org. Chem., Jpn. 2023, 81, 1028–1039.
A novel aromatic compound from the fungus Synnemellisia sp. FKR-0921.
A. Tahara, K. Tani, M. Wakatsuki, T. Tokiwa, M. Higo, K. Nonaka, T. Hirose, R. Hokari, A. Ishiyama, M. Iwatsuki, Y. Watanabe, M. Honsho, Y. Asami, H. Matsui, T. Sunazuka, H. Hanaki, T. Teruya, T. Ishii.
J. Antibiot., 2023, 76, 706-710.
Medermycin inhibits TNFα-promoted inflammatory reaction in human synovial fibroblasts.
S. Inoue, Y. Inahashi, M. Itakura, G. Inoue, K. Muneshige, T. Hirose, M. Iwatsuki, M. Takaso, M. Miyagi, K. Uchida.
Int. J. Mol. Sci., 2023, 24, 13871.
Two-phase terpenoid biosynthesis-inspired divergent total syntheses of bridged polycyclic natural products
テルペノイド二相系生合成に学ぶ架橋多環式天然物の網羅的全合成
Deep generative model of constructing chemical latent space for small to large molecular structures with 3D complexity.
T. Ochiai, T. Inukai, M. Akiyama, K. Furui, M. Ohue, N. Matsumori, S. Inuki, M. Uesugi, T. Sunazuka, K. Kikuchi, H. Kakeya, Y. Sakakibara.
ChemRxiv., 2023, 1-39.
A combination strategy of a semisynthetic macrolide, 5-O-mycaminosyltylonolide with polymyxin B nonapeptide for multi-drug resistance P. aeruginosa.
A. Kimishima, K. Sakai, M. Honsho, H. Matsui, P. Wasuwanich, Y. Watanabe, M. Iwatsuki, T. Sunazuka, N. Arima, K. Abe, H. Hanaki, Y. Asami.
J. Antibiot., 2023, 76, 499-501.
Development of a nitrogen-bound hydrophobic auxiliary: application to solid/hydrophobic tag relay synthesis of calpinactam.
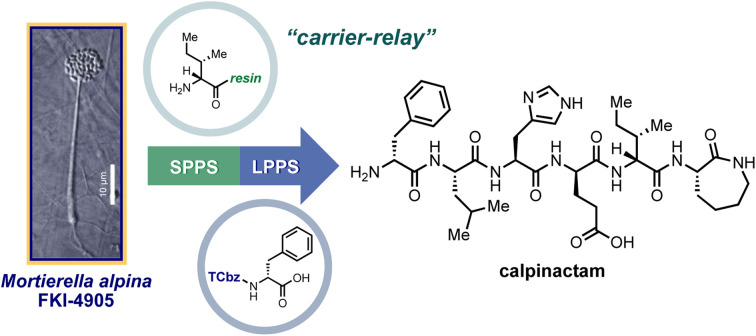
H. Nakahara, G. Sennari, Y. Noguchi, T. Hirose, T. Sunazuka.
Chem. Sci., 2023, 14, 6882-6889.
New antimalarial fusarochromanone analogs produced by the fungal strain Fusarium sp. FKI-9521
Y. Watanabe, E. Arakawa, N. Kondo, K. Nonaka, A. Ikeda, T. Hirose, T. Sunazuka, R. Hokari, A. Ishiyama, M. Iwatsuki.
J. Antibiot., 2023, 76, 384-391.
An efflux pump deletion mutant enabling the discovery of a macrolides as an overlooked anti-P. aeruginosa active compound.
A. Kimishima, K. Sakai, M. Honsho, P. Wasuwanich, H. Matsui, Y. Watanabe, M. Iwatsuki, T. Sunazuka, N. Arima, K. Abe, H. Hanaki, Y. Asami.
J. Antibiot., 2023, 76, 301-303.
Studies on the catechin constituents of Bark of Cinnamomum sieboldii.
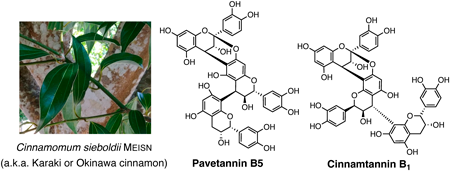
T. Hirose, K. Ozaki, Y. Saito, R. Takai-Todaka, H. Matsui, M. Honsho, M. Iwatsuki, Y. Asami, K. Katayama, T. Sunazuka, H. Hanaki, T. Teruya.
Chem. Pharm. Bull., 2023, 71, 374-379.
Hakuhybotrol, a polyketide produced by Hypomyces pseudocorticiicola, characterized with the assistance of 3D ED/MicroEd.
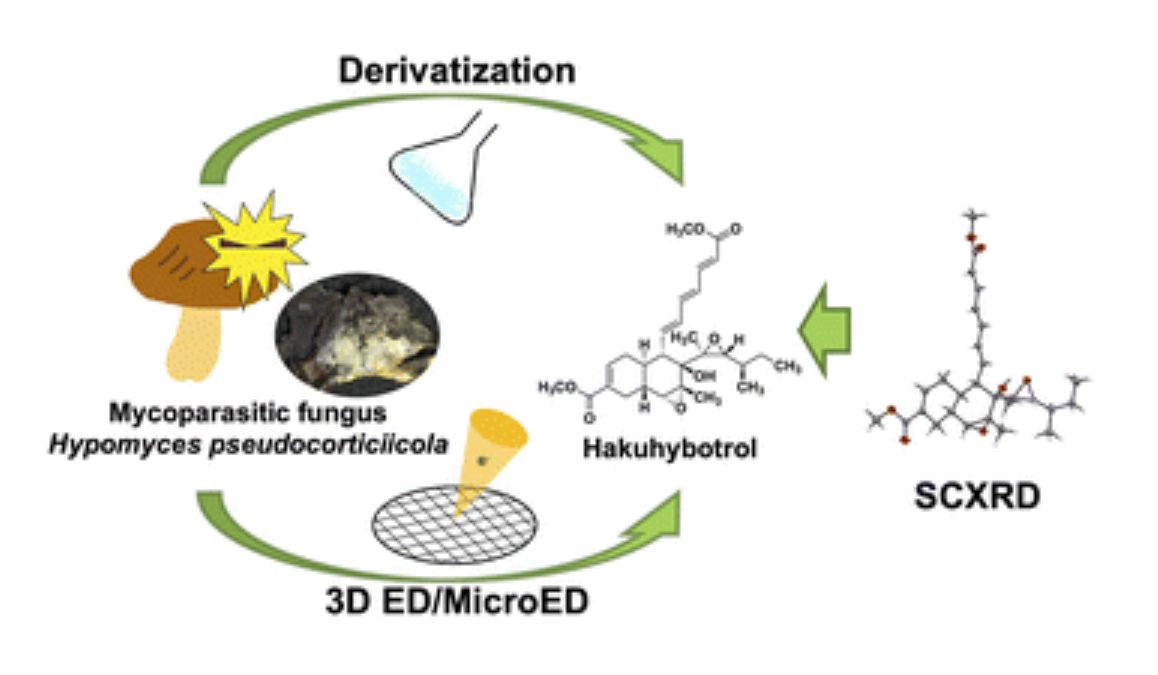
Y. Watanabe, S. Takahashi, S, Ito, T. Tokiwa, Y. Noguchi, H. Azami, H. Kojima, M. Higo, S. Ban, K. Nagai, T. Hirose, T. Sunazuka, T. Yaguchi, K. Nonaka, M. Iwatsuki.
Org. Biomol. Chem., 2023, 21, 2320-2330.
Jietacin derivative inhibits TNF-α-mediated inflammatory cytokines production via suppresion of the NF-κB pathway in synovial cells.
K. Muneshige, Y. Inahashi, M. Itakura, M. Iwatsuki, T. Hirose, G. Inoue, M. Takaso, T. Sunazuka, Y. Ohashi, E. Ohta, K. Uchida.
Pharmaceuticals, 2023, 16, 5.
A new selective inhibitor for IMP-1 metallo-β-lactamase, 3Z,5E-octa-3,5-diene-1,3,4-tricarboxylic acid-3,4-anhydride.
A. Ikeda, Y. Ikegaya, M. Honsho, H. Matsui,K. Nonaka, T. Ishii, Y. Asami, H. Hanaki, T. Hirose, T. Sunazuka.
Bioorg. Med. Chem., 2023, 78, 117109.
2022
Chemical degradation-inspired total synthesis of the antibiotic macrodiolide, luminamicin.
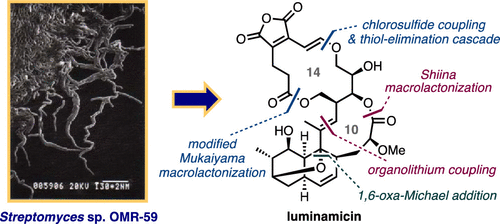
A. Kimishima, H. Ando, G. Sennari, Y. Noguchi, S. Sekikawa, T. Kojima, M. Ohara, Y. Watanabe, Y. Inahashi, H. Takada, A. Sugawara, T. Matsumaru, M. Iwatsuki, T. Hirose, T. Sunazuka.
J. Am. Chem. Soc., 2022, 144, 23148-23157.
Pot-economical synthesis of cyclic depsipeptides using a hydrophobic anchor molecule toward the construction of an unnatural peptide library.
Y. Noguchi, S. Sekikawa, Y. Nogaki, Y. Satake, N. Murashima, T. Kirisawa, G. Schiffer, J. Koebberling, T. Hirose, T. Sunazuka.
Tetrahedron, 2022, 128, 133100.
Ivermectin inhibits HBV entry into the nucleus by suppressing KPNA2.
A. Nakanishi, H. Okumura, T. Hashita, A. Yamashita, Y. Nishimura, C. Watanabe, S. Kamimura, S. Hayashi, S. Murakami, K. Ito, T. Iwao, A. Ikeda, T. Hirose, T. Sunazuka, Y. Tanaka, T. Matsunaga.
Viruses, 2022, 14, 2468.
Koshidacins A and B, antiplasmodial cyclic tetrapeptides from the Okinawa fungus Pochonia boninensis FKR-0564.
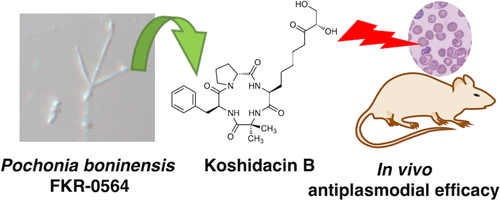
Y. Watanabe, K. Hachiya, A. Ikeda, K. Nonaka, M. Higo, R. Muramatsu, C. Noguchi, M. Honsho, Y. Asami, Y. Inahashi, T. Hirose, H. Matsui, T. Sunazuka, H. Hanaki, T. Ishii, T. Teruya, R. Hokari, A. Ishiyama, M. Iwatsuki.
J. Nat. Prod., 2022 , 85, 2641-2649.
Unified Total Syntheses of Benzenoid Cephalotane-Type Norditerpenoids: Cephanolides and Ceforalides
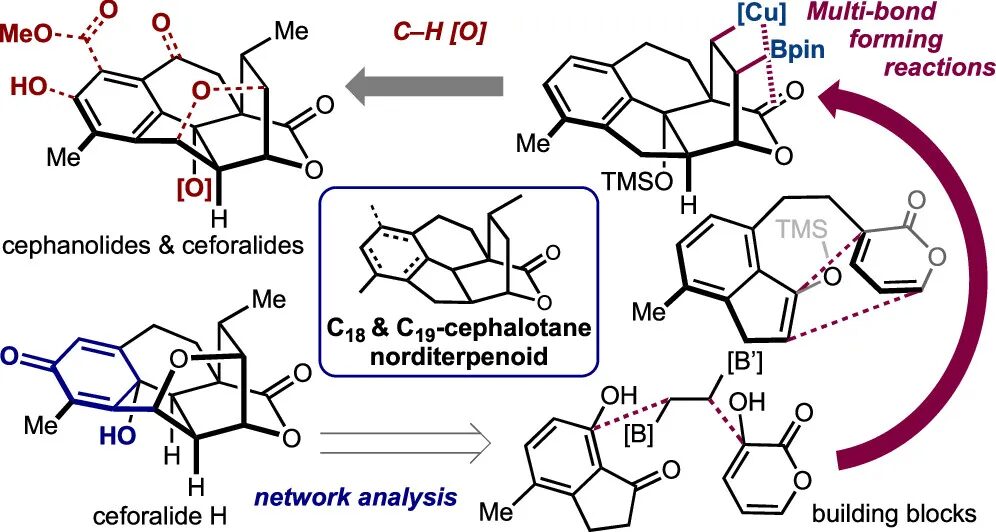
Sennari, G.; Gardner, K. E.†; Wiesler, S.†; Haider, M.; Eggert, A.; Sarpong, R.*
J. Am. Chem. Soc. 2022, 144, 19173–19185.
Strategy Evolution in a Skeletal Remodeling and C–H Functionalization-Based Synthesis of the Longiborneol Sesquiterpenoids
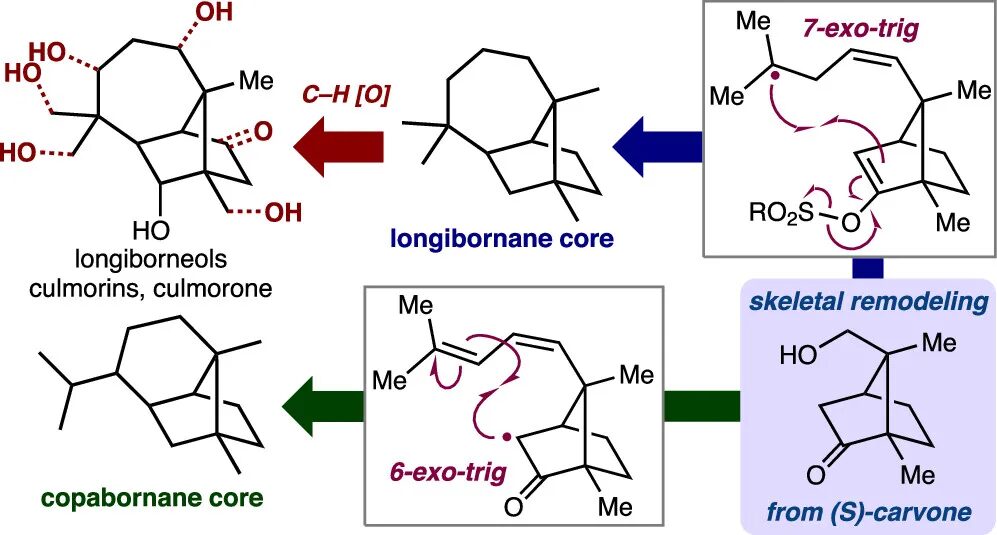
Lusi, R. F.†; Sennari, G.†,*; Sarpong, R.*
J. Am. Chem. Soc. 2022, 144, 17277–17294.
Nanaomycin E inhibits NLRP3 inflammasome activation by preventing mitochondrial dysfunction.
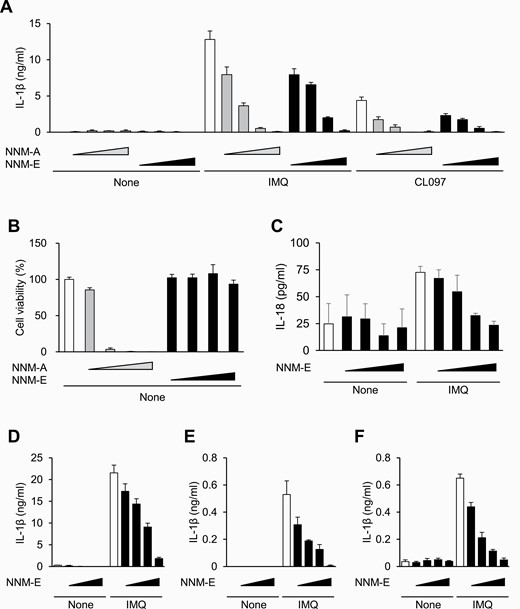
Y. Matsui, N. Takemura, Y. Shirasaki, M. Takahama, Y. Noguchi, K. Ikoma, Y. Pan, S. Nishida, M. Taura, A. Nakayama, T. Funatsu, T. Misawa, Y. Harada, T. Sunazuka, T. Saitoh.
Int. Immunol., 2022, 34, 505-517.
Fungal secondary metabolite exophilic acid selectively inhibits the entry of hepatitis B and D viruses.
C. Kobayashi, Y. Watanabe, M. Oshima, T. Hirose, M. Yamasaki, M. Iwamoto, M. Iwatsuki, Y. Asami, K. Kuramochi, K. Wakae, H. Aizaki, M. Muramatsu, C. Sureau, T. Sunazuka, K. Watashi.
Viruses, 2022, 14, 764.
Ivermection repress Wnt/β-catenin signaling by binding to TELO2, a regulator of phosphatidylinositol 3-kinase-related kinases.
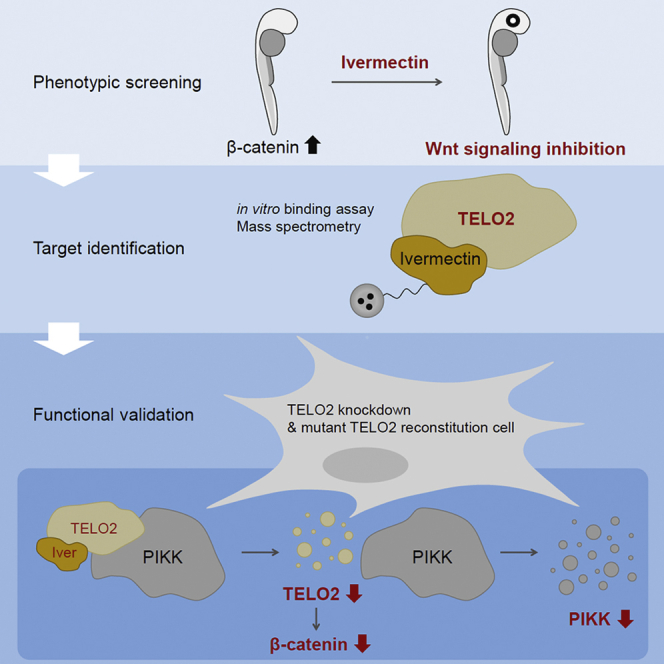
H. Yonexawa, A. Ikeda, R. Takahashi, H. Endo, Y. Sugawara, M. Goto, M. Kanno, S. Ogawa, K. Nakamura, H. Ujiie, M. Iwatsuki, T. Hirose, T. Sunazuka, Y. Uehara, N. Nishiya.
iScience, 2022, 25, 103912.
Afidopyropen, a novel insecticide originating from microbial secondary extracts.
R. Horikoshi, K. Goto, M. Mitomi, K. Oyama, T. Hirose, T. Sunazuka, S. Omura.
Sci. Rep., 2022, 12, 2827.
Hakuhybotric acid, a new antifungal polyketide produced by a mycoparasitic fungus Hypomyces pseudocorticiicola FKI-9008.
Y. Watanabe, Y. Yoshida, T. Tokiwa, M. Higo, S. Ban, A. Ikeda, Y. Noguchi, T. Hirose, K. Nonaka, T. Yaguchi, M. Iwatsuki.
J. Gen. Appl. Microbiol., 2022, 68, 200-206.
2021
In situ click chemistry and omura matural products drug discovery research.
Sesquicillin F, a new insectcidal meroterpenoid produced by Mariannaea macrochlamydospora FKI-4735.
K. Sakai, M. Iwatsuki, T. Kaneta, A. Kimishima, Y. Asami, T. Sunazuka, R. Masuma, K. Nonaka.
J. Antibiot., 2021, 74, 817-820.
Total Syntheses and Chemical Biology Studies of Hymeglusin and Fusarilactone A, Novel Circumventors of β‐Lactam Drug Resistance in Methicillin‐Resistant Staphylococcus aureus.
M. Kanaida, A. Kimishima, S. Eguchi, M. Iwatsuki, Y. Watanabe, M. Honsho, T. Hirose, Y. Noguchi, K. Nonaka, G. Sennari, H. Matsui, C. Kaito, H. Hanaki, Y. Asami, T. Sunazuka.
Chem. Med. Chem., 2021 , 16, 2106-2111.
Discoveries and syntheses of highly potent antimalarial troponoids.
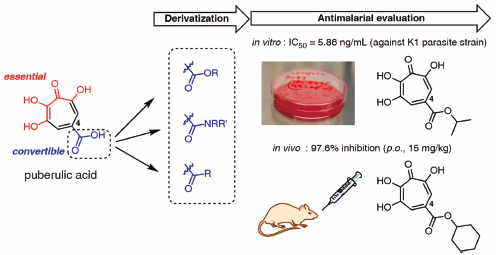
R. Saito, G. Sennari, A. Nakajima, A. Kimishima, M. Iwatsuki, A. Ishiyama, R. Hokari, T. Hirose, T. Sunazuka.
Chem. Pharm. Bull., 2021, 69, 6, 564-572
Pd-catalyzed regio- and stereoselective hydrostannylation of an alkyl ethynyl ether/one-pot stille coupling enables the synthesis of 14-membered macrolactone of luminamicin.
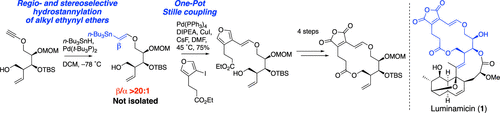
A. Sugawara, H. Takada, T. Hirose, A. Kimishima, T. Yamada, M. Toda, T. Kojima, T. Matsumaru, T. Sunazuka.
Org. Lett., 2021, 23, 1758-1763.
Structure, solubility, and permeability relationship in a diverse middle molecule library.
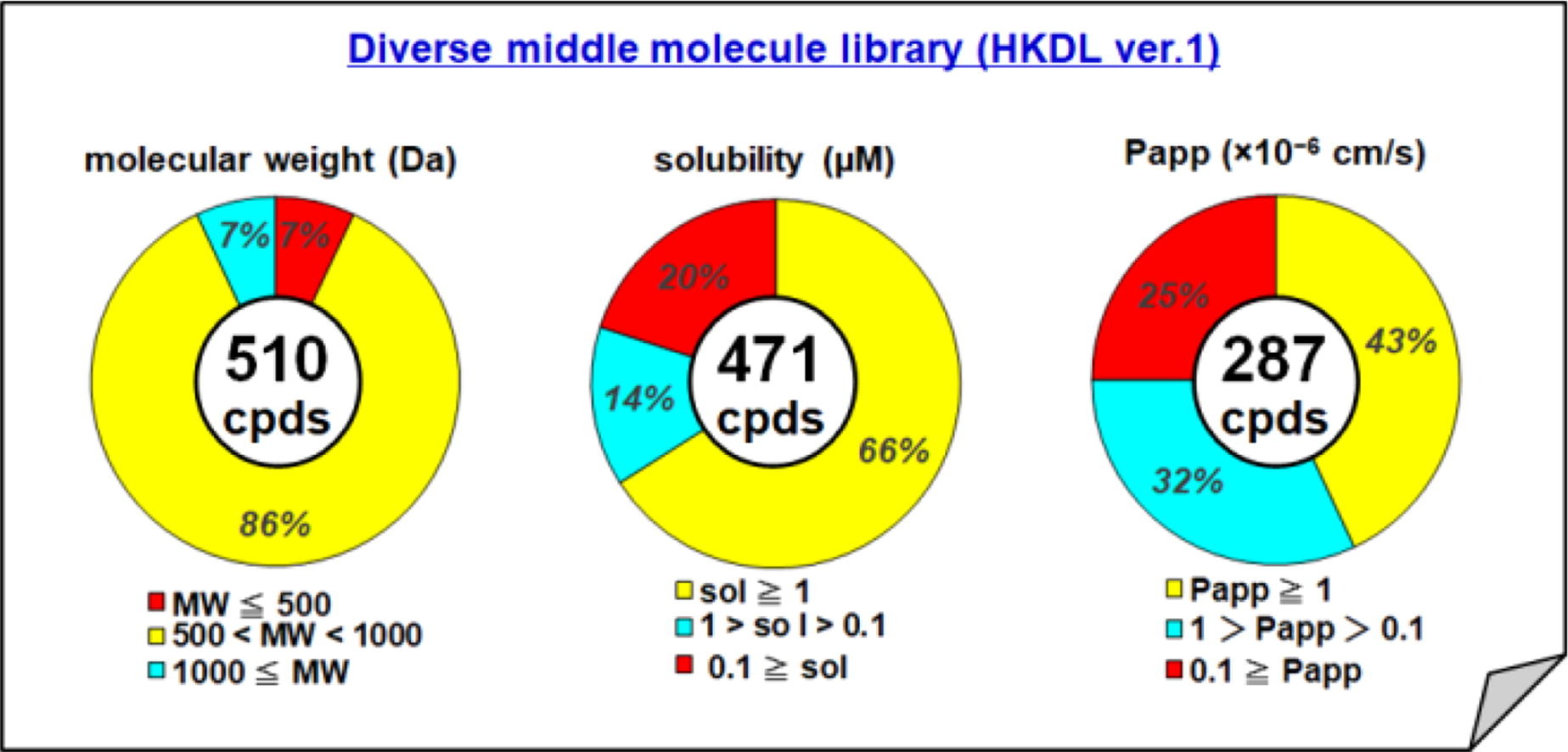
H. Miyachi, K. Kanamitsu, M. Isii, E. Watanabe, A. Katsuyama, S. Otsuguro, F. Yakushiji, M. Watanabe, K. Matsui, Y. Sato, S. Shuto, T. Tadokoro, S. Kita, T. Matsumaru, A. Masuda, T. Hirose, M. Iwatsuki, Y. Shigeta, T. Nagano, H. Kojima, S. Ichikawa, T. Sunazuka, K. Maenaka T. Sunazuka.
Bioorg. Med. Chem. Lett., 2021, 37, 127847
Toward the total synthesis of aurodox: preparation of the key hemiacetal-lactone.
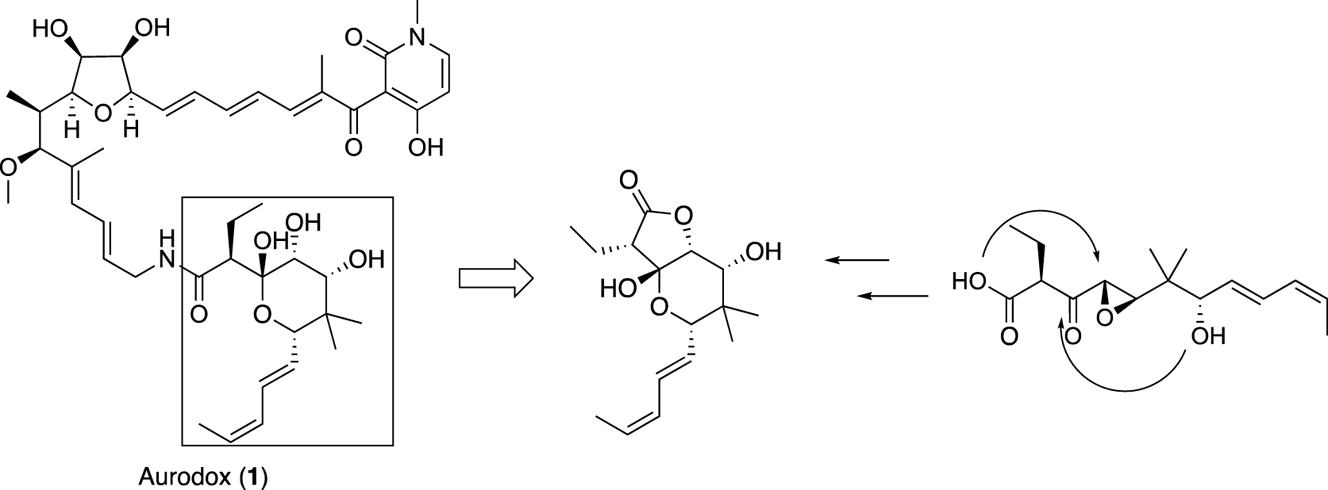
M. Ohara, A. Ikeda, A. Nakajima, T. Ono, Y. Noguchi, A. Watanabe, T. Hirose, T. Sunazuka
Tetrahedron Lett., 2021, 66, 152799.
Unified enantioselective total synthesis of 3,6-dioxygenated diketopiperadine natural products, diatretol and lepistamides A, B and C.
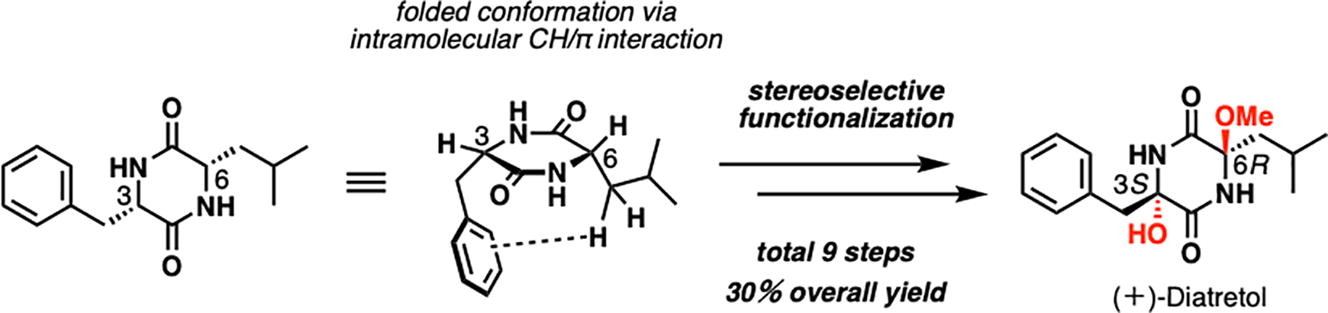
S. Takahashi, A. Kimishima, T. Hirose, T. Yamada, A. Sugawara, T. Shirahata, Y. Noguchi, M. Iwatsuki, R. Hokari, A. Ishiyama, Y. Kobayashi, T. Sunazuka
Tetrahedron Lett., 2021, 67, 152895.
2020
Absolute structure and anti-oxidative activity of chaetochiversin C isolated from fungal strain sp. FKI-7792 by phycochemical screening.
H. Matsuo, T. Hirose, T. Mokudai, K. Nonaka, Y. Niwano, T. Sunazuka, Y. Takahashi, S. Ōmura, T. Nakashima.
J. Gen. Appl. Microbiol., 2020, 66, 181-187
Nanaomycin K, a new epithelial-mesenchymal transition inhibitor produced by the actinomycete “Streptomyces rosa subsp. notoensis” OS-3966
H. Matsuo, J. Nakanishi, Y. Noguchi, K. Kitagawa, K. Shigemura, T. Sunazuka, Y. Takahashi, S. Ōmura, T. Nakashima.
J. Biosci. Bioeng., 2020, 129 (3), 291-295
Thioporidiols A and B: Two New Sulfur Compounds Discovered by Molybdenum-Catalyzed Oxidation Screening from Trichoderma polypori FKI-7382
H. Matsuo, Y. Noguchi, R. Miyano, M. Higo, K. Nonaka, T. Sunazuka, Y. Takahashi, S. Ōmura, T. Nakashima.
Antibiotics, 2020, 9, 236-244
The Nonantibiotic Macrolide EM900 Attenuates House Dust Mite-Induced Airway Inflammation in a Mouse Model of Obesity-Associated Asthma
H. Sadamatsu, K. Takahashi, H. Tashiro, Y. Kurihara, G. Kato, M. Uchida, Y. Noguchi, K. Kurata, S. Ōmura, S. Kimura, T. Sunazuka, N. Sueoka-Aragane.
Int. Arch. Allergy Immunol, 2020, 181, 665-674
Tihoporidiols A and B: Two New Sulfur Compounds Discovered by Molybdenum-Catalyzed Oxidation Screening from Trichoderma polypori FKI-7382.
H. Matsuo, Y. Noguchi, R. Miyano, M. Higo, K. Nonaka, T. Sunazuka, S. Ōmura, T. Nakashima
Antibiotics, 2020, 9, 236-243
Efficient synthesis of a ryanodine binding inhibitor verticilide using two practical approaches
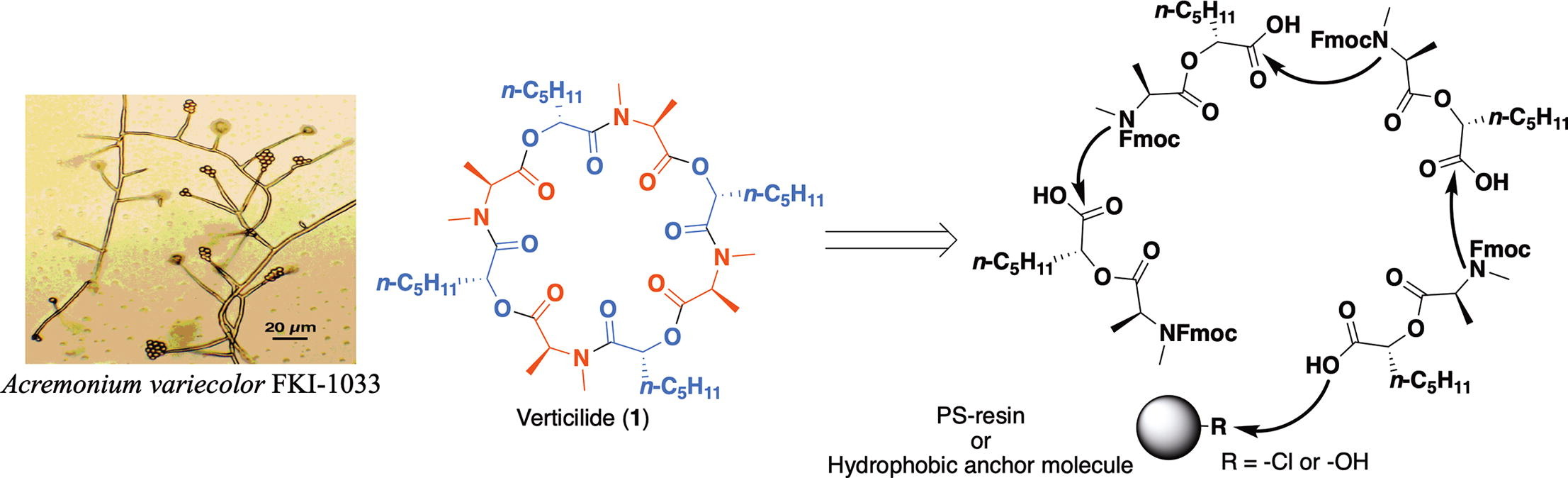
A. Watanabe, Y. Noguchi, T. Hirose, S. Monma, Y. Satake, T. Arai, K. Masuda, N. Murashima, K. Shiomi, S. Ōmura, T. Sunazuka
Tetrahedron Lett., 2020, 61, 13, 151699
Rapid Exploration of Novel Anthelmintic Agents from Alkyne-Bearing Avermectin Derivatives via Click Chemistry.
T. Hirose, A. Ikeda, A. Tsutsui, S. Ōmura, T. Sunazuka
Heterocycles, 2020, 101, 116-125
2019
Trichothioneic acid, a new antioxidant compound produced by the fungal strain Trichoderma virens FKI-7573.
R. Miyano, H. Matsuo, T. Mokudai, Y. Noguchi, M. Higo, K. Nonaka, Y. Niwano, T. Sunazuka, K. Shiomi, Y. Takahashi, S. Ōmura, T. Nakashima.
J. Biosci. Bioeng., 2019, doi: 10.1016/j.jbiosc.2019.11.007
The non-antibiotic macrolide EM900 attenuates HDM and poly(I:C)-induced airway inflammation with inhibition of macrophages in a mouse model.
H. Sadamatsu, K. Takahashi, H. Tashiro, G. Kato, Y. Noguchi, K. Kurata, S. Ōmura, S. Kimura, T. Sunazuka, N. Sueoka-Aragane.
Inflamm. Res., 2020, 69, 139-151
Synthesis of pyripyropene derivatives and their pest-control efficacy.
K. Goto, R. Horikoshi, S. Nakamura, M. Mitomi, K. Oyama, T. Hirose, T. Sunazuka, S. Ōmura.
J. Pestic Sci., 2019, 25, 255-263.
Absolute structure and anti-oxidative activity of chaetochiversin C isolated from fungal strain Neocosmospora sp. FKI-7792 by physicochemical screening
H. Matsuo, T. Hirose, T. Mokudai, K. Nonaka, Y. Niwano, T. Sunazuka, Y. Takahashi, S, Ōmura, T. Nakashima.
J. Gen. Appl. Microbiol., 2019, Aug 26;66(3):181-187.
Nanaomycin K, a new epithelial-mesenchymal transition inhibitor produced by the actinomycete “Streptomyces rosa subsp. notoensis” OS-3966.
H. Matsuo, J. Nakanishi, Y. Noguchi, K. Kitagawa, K. Shigemura, T. Sunazuka, Y. Takahashi, S. Ōmura, T. Nakashima
J. Biosci. Bioeng., 2019, 2127, 1389-1723.
Synthesis of the Antimalarial Peptide Aldehyde, a Precursor of Kozupeptin A, Utilizing a Designed Hydrophobic Anchor Molecule.
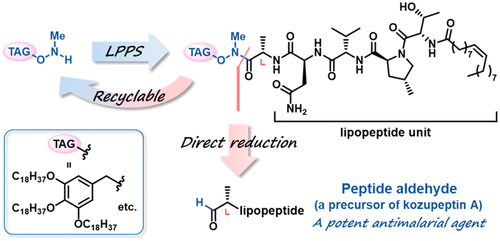
Y. Hayashi, T. Hirose, M. Iwatsuki, S. Ōmura, T. Sunazuka.
Org. Lett., 2019, 21, 8229-8233.
Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A(H1N1)pdm09 virus infection by interfering with virus internalization process
D. H. Tran, R. Sugamata, T. Hirose, S. Suzuki, Y. Noguchi, A. Sugawara, F. Ito, T. Yamamoto, S. Kawachi, K. S. Akagawa, S. Ōmura, T. Sunazuka, N. Ito, M. Mimaki, K. Suzuki.
J. Antibiot., 2019, 72, 759-768.
Jietacins, azoxy natural products, as novel NF-κB inhibitors: Discovery, synthesis, biological activity, and mode of action
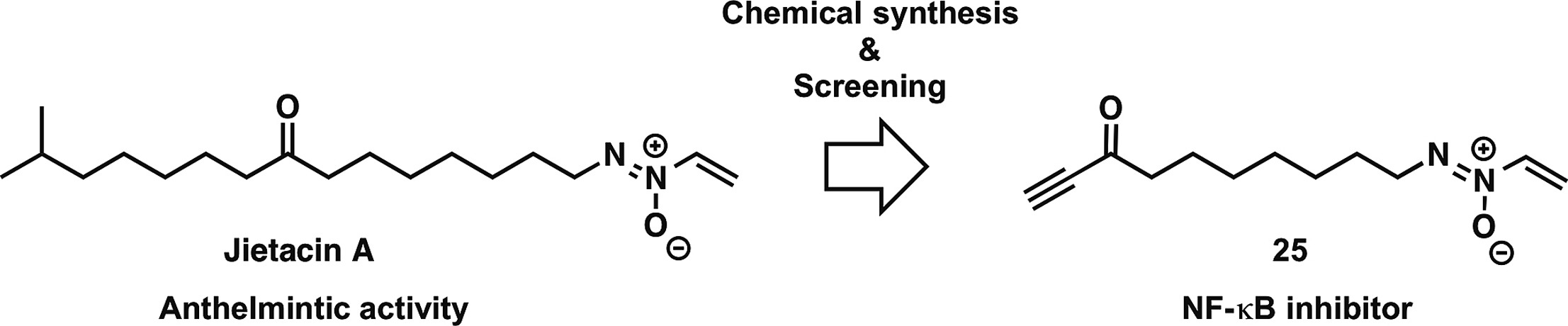
M. Watanabe, A. Sugawara, Y. Noguchi, T. Hirose, S. Ōmura, T. Sunazuka, R. Horie.
Eur. J Med. Chem., 2019, 178, 636-647.
Synthesis and insecticidal efficacy of pyripyropene derivatives. Part II-Invention of afidopyropen.
K. Goto, R. Horikoshi, M. Mitomi, K. Oyama, T. Hirose, T. Sunazuka, S. Ōmura.
Y.J. Antibiot., 2019, 72. 661-681.
Fusaramin, an antimitochondrial compound produced by Fusarium sp., discovered using multidrug-sensitive Saccharomyces cerevisiae.
K. Sakai, Y. Unten, M. Iwatsuki, H. Matsuo, W. Fukasawa, T. Hirose, T. Chinen, K. Nonaka, T. Nakashima, T. Sunazuka, T. Usui, M. Murai, H. Miyoshi, Y. Asami, S. Ōmura, K. Shiomi.
J. Antibiot., 2019, 72, 645-652.
Erythromycin acts through the ghrelin receptor to attenuate inflammatory responses in chondrocytes and maintain joint integrity.
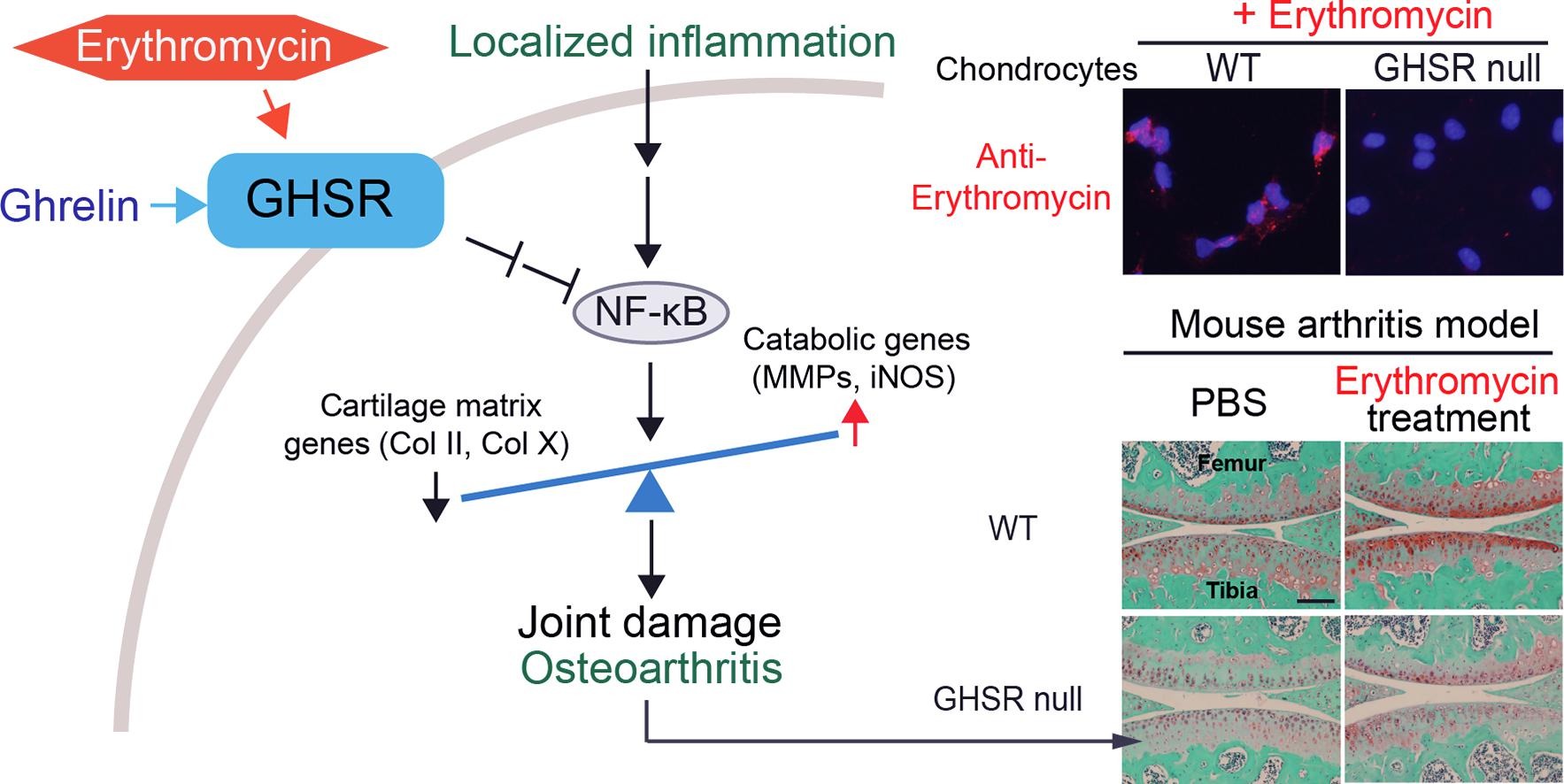
T. Uchimura, D. S. Nakamura, E. M. Link, Y. Noguchi, S. Ōmura, T. Sunazuka, D. J. Greenblatt, L. Zeng.
Biochem. Pharmacol., 2019, 165, 79-90.
Nanaomycin I and J: New nanaomycins generated by mycothiol-mediated compounds from “Streptomyces rosa subsp. notoensis” OS-3966.
Matsuo H, Noguchi Y, Takè A, Nakanishi J, Shigemura K, Sunazuka T, Takahashi Y, Ōmura S, Nakashima T.
J. Biosci. Bioeng. 2019, 127, 549-553.
Kozupeptins, Antimalarial Agents Produced by Paracamarosporium Species: Isolation, Structural Elucidation, Total Synthesis, and Bioactivity.
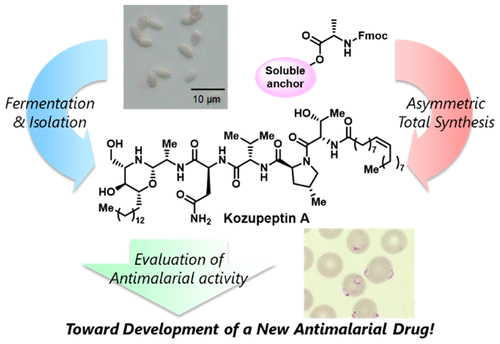
Yumi Hayashi, Wataru Fukasawa, Tomoyasu Hirose, Masato Iwatsuki, Rei Hokari, Aki Ishiyama, Masahiro Kanaida, Kenichi Nonaka, Akira Take, Kazuhiko Otoguro, Satoshi Ōmura, Kazuro Shiomi, and Toshiaki Sunazuka
Org. Lett. 2019, 21, 7, 2180-2184.
2018
Anti-Inflammatory Effects of EM900 on Cultured Human Nasal Epithelial Cells
Nozomu Wakayama, Shoji Matsune, Eriko Takahara, Kuwon Seine, Yuma Yoshioka, Mariko Ishida, Satoshi Yamguchi, Kimihiro Okubo, Toshiaki Sunazuka, Satoshi Ōmura.
J. Nippon Med. Sch., 2018, 85, 265-270.
Pestynol, an Antifungal Compound Discovered Using a Saccharomyces cerevisiae 12geneΔ0HSR-iERG6-Based Assay
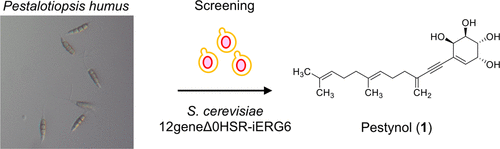
Katsuyuki Sakai, Tomoyasu Hirose, Masato Iwatsuki, Takumi Chinen, Toru Kimura, Takuya Suga, Kenichi Nonaka, Takuji Nakashima, Toshiaki Sunazuka, Takeo Usui, Yukihiro Asami, Satoshi Ōmura, and Shiomi Kazuro.
J. Nat. Prod., 2018, 81, 1604–1609.
Insecticidal properties of pyripyropene A, a microbial secondary metabolite, against agricultural pests
Ryo Horikoshi, Kimihiko Goto, Tomoyasu Hirose, Masaaki Miomi, Masato Iwatsuki, Kazuhiko Oyama, Toshiaki Sunazuka, and Satoshi Ōmura.
J. Pestic. Sci., 2018, 43, 266–271.
Synthesis and insecticidal efficacy of pyripyropene derivatives focusing on the C-1, C-7, and C-11 positions’ substituent groups
Goto Kimihiko, Horikoshi Ryo, Mitomi Masaaki, Oyama Kazuhiko, Tomoyasu Hirose, Satoshi Ōmura, and Toshiaki Sunazuka.
J. Antibiot. 2018, 71, 785–797.
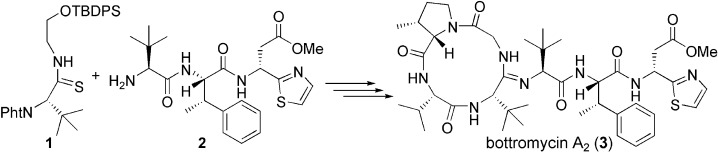
Synthesis and evaluation of antibacterial activity of Bottromycins
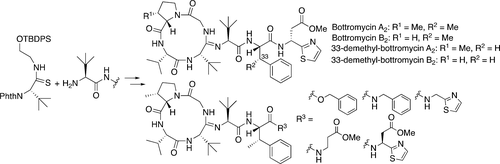
Takeshi Yamada, Miu Yagita, Yutaka Kobayashi, Goh Sennari, Hidehito Matsui, Yuki Horimatsu, Hideaki Hanaki, Tomoyasu Hirose, Satoshi Ōmura, and Toshiaki Sunazuka.
J. Org. Chem. 2018, 83(13):7135-7149.
Jietacins, azoxy antibiotics with potent nematocidal activity: Design, synthesis, and biological evaluation against parasitic nematodes
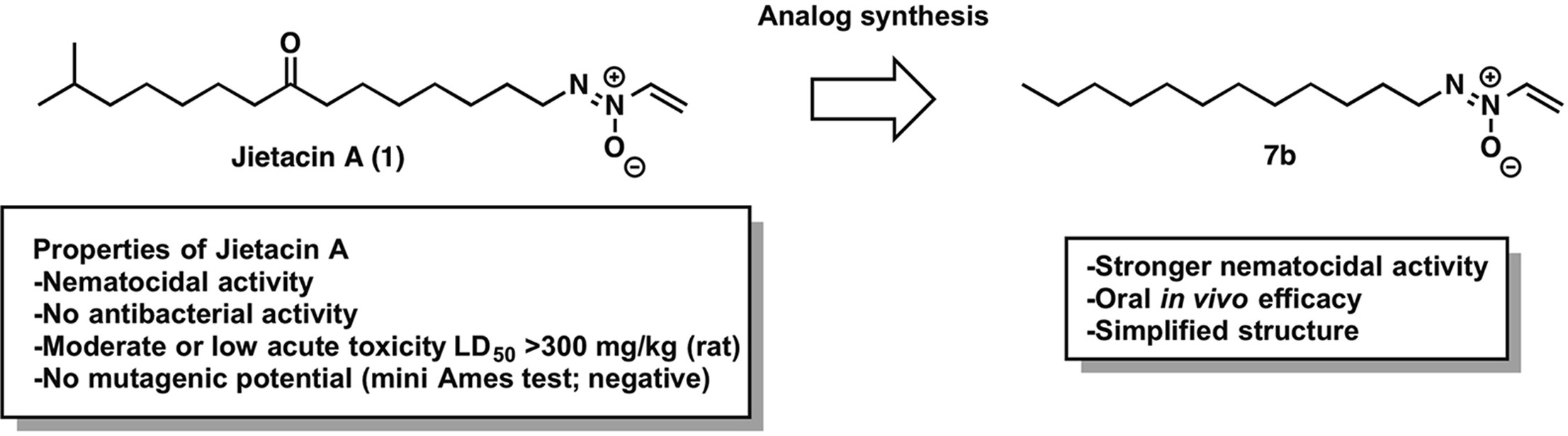
Akihiro Sugawara, Masahiko Kubo, Tomoyasu Hirose, Kyoichi Yahagi, Noriaki Tsunoda, Yoshihiko Noguchi, Takuji Nakashima, Yoko Takahashi, Claudia Welz, Dennis Mueller, Christina Mertens, Johannes Kobberling, Satoshi Ōmura and Toshiaki Sunazuka.
Eur. J. Med. Chem. 2018, 145, 524-538.
Toward the total synthesis of luminamicin; an anaerobic antibiotic: construction of highly functionalized cis-decalin containing a bridged ether moiety.
Hiroyasu Ando, Aoi Kimishima, Motoyoshi Ohara, Tomoyasu Hirose, Takanori Matsumaru, Hirokazu Takada, Keisuke Morodome, Takehiro Miyamoto, Akihiro Sugawara, Satoshi Ōmura and Toshiaki Sunazuka.
J. Antibiot. 2018, 71, 268-272.
2017
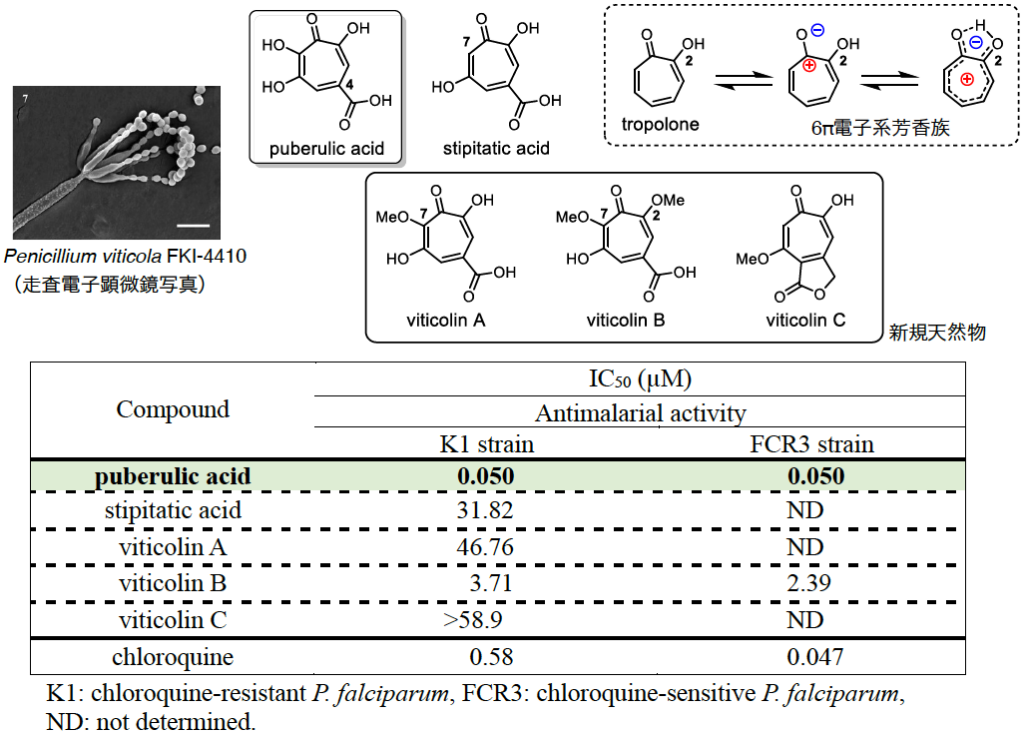
Antimalarial troponoids, puberulic acid and viticolins; divergent synthesis and structure-activity relationship studies.
Goh Sennari, Ryo Saito, Tomoyasu Hirose, Masato Iwatsuki, Aki Ishiyama, Rei Hokari, Kazuhiko Otoguro, Satoshi Ōmura and Toshiaki Sunazuka.
Sci. Rep. 2017 Aug 3;7(1):7259.
Stereo- and substituent-enabled divergent synthesis of 5,6-spiroketal analogs of avermectin containing a triazole function.
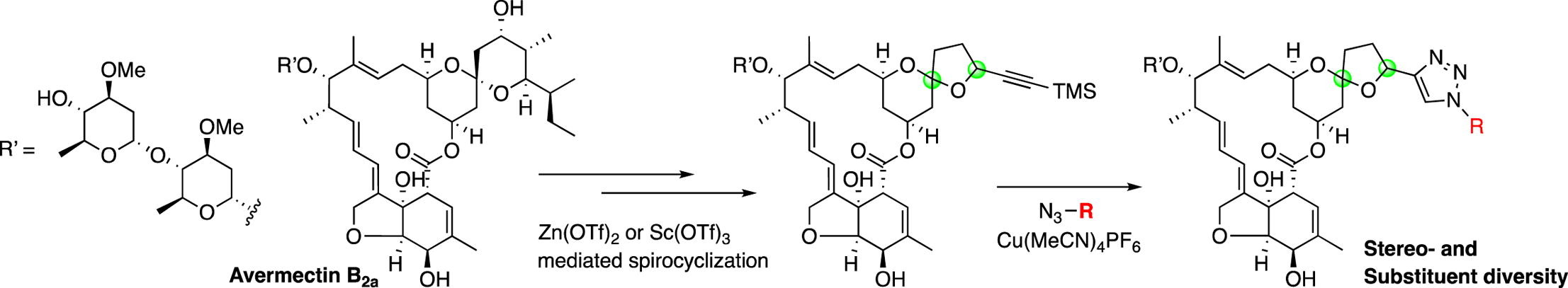
Takeshi Yamada, Yuki Horimatsu, Tomoyasu Hirose, Akihiro Sugawara, Satoshi Ōmura and Toshiaki Sunazuka.
Tetrahedron Lett. 2017, 58, 3119-3124.
5-O-Mycaminosyltylonolide antibacterial derivatives: design, synthesis and bioactivity.
Akihiro Sugawara, Hitomi Maruyama, Sho Shibusawa, Hidehito Matsui, Tomoyasu Hirose, Ayumi Tsutsui, Robrecht Froyman, Carolin Ludwig, Johannes Koebberling, Hideaki Hanaki, Gerd Kleefeld, Satoshi Ōmura and Toshiaki Sunazuka.
J. Antibiot. 2017, 70, 878-887.
Ivermectin efficacy against Biomphalaria, intermediate host snail vectors of Schistosomiasis.
Naftale Katz, Neusa Araujo, Paulo Marcos Zech Coelho, Carlos Medicis Morel, Ana Rosa Linde-Arias, Takeshi Yamada, Yuki Horimatsu, Koh Suzuki, Satoshi Ōmura and Toshiaki Sunazuka
J. Antibiot. 2017, 70, 680-684.
Total synthesis of (±)-naphthacemycin A9, possessing both antibacterial activity against methicillin-resistant Staphylococcus aureus and circumventing effect of β-lactam resistance
Tomoyasu Hirose, Yasuhiro Kojima, Hidehito Matsui, Hideaki Hanaki, Masato Iwatsuki, Kazuro Shiomi, Satoshi Ōmura and Toshiaki Sunazuka
J. Antibiot. 2017, 70, 574-581.
Naphthacemycins, novel circumventors of β-lactam resistance in MRSA, produced by Streptomyces sp. KB-3346-5. II. Structure elucidation.
Atsushi Fukumoto, Yong-Pil Kim, Masato Iwatsuki, Tomoyasu Hirose, Toshiaki Sunazuka, Hideaki Hanaki, Satoshi Ōmura and Kazuro Shiomi.
J. Antibiot. 2017, 70, 568-573.
Identification of pyripyropene A as a promising insecticidal compound in a microbial metabolite screening.
Ryo Horikoshi, Kimihiko Goto, Masaaki Mitomi, Kazuhiko Oyama, Toshiaki Sunazuka and Satoshi Ōmura
J. Antibiot. 2017, 70, 272-276.
Total Synthesis and Determination of the Absolute Configuration of Naturally Occurring Mangromicin A, with Potent Antitrypanosomal Activity.
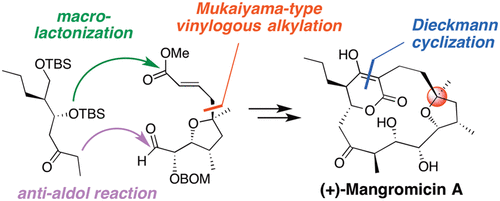
Hirokazu Takada, Takeshi Yamada, Tomoyasu Hirose, Takuma Ishihara, Takuji Nakashima, Yo̅ko Takahashi, Satoshi Ōmura and Toshiaki Sunazuka
Org. Lett. 2017, 19, 230-233.
2016
Rapid Identification via In Situ Click Chemistry of a Novel Chitinase Inhibitor
Tomoyasu Hirose, Toshiaki Sunazuka and Satoshi Ōmura
J. Synth. Org. Chem., Jpn. 2016, 74, 1090-1097.
The Design, Synthesis, and Evaluation of 1,5,7-Trisubstituted-3-Pyridyl-Xanthones for Use as Insecticides Starting from Pyripyropene A
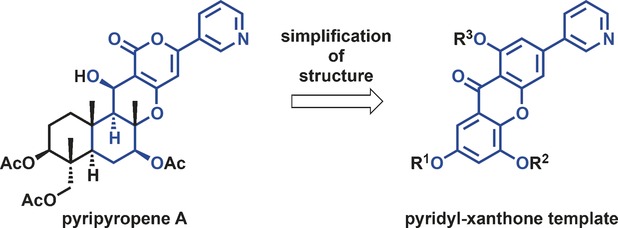
Shinichiro Fuse, Keisuke Matsumura, Kohei Johmoto, Hidehiro Uekusa, Hiroshi Tanaka, Tomoyasu Hirose, Toshiaki Sunazuka, Satoshi Ōmura and Takashi Takahashi
Chem. Eur. J. 2016, 22, 18450-18455.
Narrow-spectrum inhibitors targeting an alternative menaquinone biosynthetic pathway of Helicobacter pylori.
Yamamoto T, Matsui H, Yamaji K, Takahashi T, Øverby A, Nakamura M, Matsumoto A, Nonaka K, Sunazuka T, Ōmura S, Nakano H.
J. Infect. Chemother. 2016, 22, 587-592.
Synthesis and stereochemical determination of an antiparasitic pseudo-aminal type monoterpene indole alkaloid.
Noguchi Y, Hirose T, Ishiyama A, Iwatsuki M, Otoguro K, Sunazuka T and Ōmura S.
J. Nat. Med. 2016, 70, 302-317.
In situ Click Chemistry for the Identification of a Potent D-Amino Acid Oxidase Inhibitor
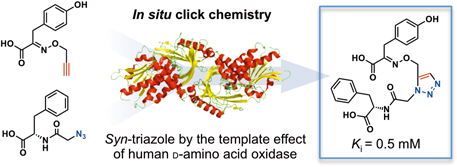
Shohei Toguchi, Tomoyasu Hirose, Kazuko Yorita, Kiyoshi Fukui, K. Barry Sharpless, Satoshi Ōmura, Toshiaki Sunazuka
Chem. Pharm. Bull. 2016, 64, 695-703.
Organocatalytic Site-Selective Acylation of Avermectin B2a, a Unique Endectocidal Drug
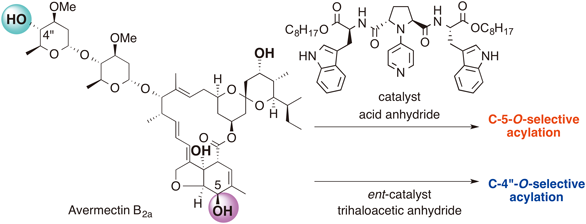
Takeshi Yamada, Koh Suzuki, Tomoyasu Hirose, Takumi Furuta, Yoshihiro Ueda, Takeo Kawabata, Satoshi Ōmura and Toshiaki Sunazuka
Chem. Pharm. Bull, 2016, 64, 856-864.
Organocatalytic Site-Selective Acylation of 10-Deacetylbaccatin III
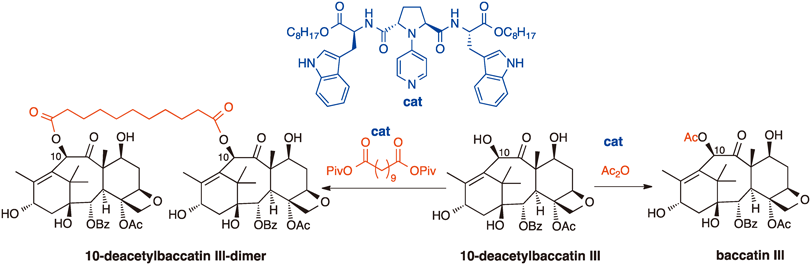
Masanori Yanagi, Ryo Ninomiya, Yoshihiro Ueda, Takumi Furuta, Takeshi Yamada, Toshiaki Sunazuka and Takeo Kawabata
Chem. Pharm. Bull, 2016, 64, 907-912.
Towards the total synthesis of the anti-trypanosomal macrolide, Actinoallolides; construction of a key linear intermediate
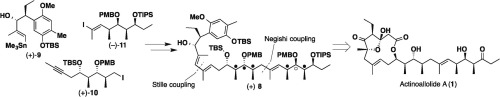
Jun Oshita, Yoshihiko Noguchi, Akito Watanabe, Goh Sennari, Shogo Sato, Tomoyasu Hirose, Daiki Oikawa, Yuki Inahashi, Masato Iwatsuki, Aki Ishiyama, Satoshi Ōmura and Toshiaki Sunazuka
Tetrahedron Lett. 2016, 57, 357-360.
Macrolides sensitize EGFR-TKI-induced non-apoptotic cell death via blocking autophagy flux in pancreatic cancer cell lines.
Shuntaro Mukai, Shota Moriya, Masaki Hiramoto, Hiromi Kazama, Hiroko Kokuba, Xiao-Fang Che, Tomohisa Yokoyama, Satoshi Sakamoto, Akihiro Sugawara, Toshiaki Sunazuka, Satoshi Ōmura, Hiroshi Handa, Takao Itoi and Keisuke Miyazawa
Int. J. Clin. Oncol. 2016, 48, 45-54.
Non-antibiotic 12-membered macrolides: design, synthesis and biological evaluation in a cigarette-smoking model.
Invited article for Professor Amos B Smith, III special issue on the occasion of 50 years of research and education in the areas of Natural Product Total Synthesis, Bioorganic and Medicinal Chemistry.
Sugawara, A; Shima, H; Sueki, A; Hirose, T; Matsui, H; Nakano, H; Hanaki, H; Akagawa, S. K; Ōmura, S and Sunazuka T.
J. Antibiot. 2016, 69, 319-326.
2015
The non-antibiotic macrolide EM900 inhibits rhinovirus infection and cytokine production in human airway epithelial cells
Nadine Lusamba Kalonji, Kazuhiro Nomura, Tetsuaki Kawase, Chiharu Ota, Hiroshi Kubo, Takeya Sato, Teruyuki Yanagisawa, Toshiaki Sunazuka, Satoshi Ōmura and Mutsuo Yamaya
Physiological Reports 2015, 3, e12557.
Asymmetric Total Synthesis of Indole Alkaloids Containing An Indoline Spiroaminal Framework.
Takeshi Yamada, Tetsuya Ideguchi-Matsushita, Tomoyasu Hirose, Tatsuya Shirahata, Rei Hokari, Aki Ishiyama, Masato Iwatsuki, Akihiro Sugawara, Yoshinori Kobayashi, Kazuhiko Otoguro, Satoshi Ōmura and Toshiaki Sunazuka
Chem. E. J. 2015, 21, 11855-11864.
Creation of customised bioactivity within a 14-membered macrolide scaffold: design, synthesis, and biological evaluation using a family-18 chitinase.
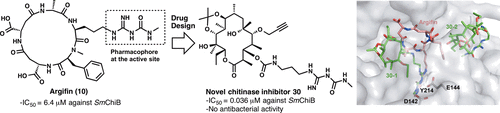
Akihiro Sugawara, Nobuo Maita, Hiroaki Gouda, Tsuyoshi Yamamoto, Tomoyasu Hirose, Saori Kimura, Yoshifumi Saito, Hayato Nakano, Takako Kasai, Hirofumi Nakano, Kazuro Shiomi, Shuichi Hirono, Takeshi Watanabe, Hisaaki Taniguchi, Satoshi Ōmura and Toshiaki Sunazuka
J. Med. Chem. 2015, 58, 4984–4997.
Macrolides promote CCL2-mediated macrophage recruitment and clearance of nasopharyngeal pneumococcal colonies in mice.
Naoki Iwanaga, Shigeki Nakamura, Kazuhiro Oshima, Toshiki Kajihara, Takahiro Takazono, Taiga Miyazaki, Koichi Izumikawa, Katsunori Yanagihara, Akihiro Sugawara, Toshiaki Sunazuka, Satoshi Ōmura and Shigeru Kohno
J. Infect. Dis. 2015, 212, 1150-1159.
Anti-inflammatory effects of a novel non-antibiotic macrolide, EM900, on mucus secretion of airway epithelium.
Ichiro Tojima, Shino Shimizu, Takao Ogawa, Hideaki Kouzaki, Satoshi Ōmura, Toshiaki Sunazuka and Takeshi Shimizu
Auris Nasus Larynx 2015, 42, 332-336.
Cinatrins D and E, and virgaricin B, three novel compounds produced by a fungus, Virgaria boninensis FKI-4958
Takahiro Ishii, Kenichi Nonaka, Akihiro Sugawara, Masato Iwatsuki, Rokuro Masuma, Tomoyasu Hirose, Toshiaki Sunazuka, Satoshi Ōmura and Kazuro Shiomi
J. Antibiot. 2015, 68, 633-637.
Jietacins with potent nematocidal activity; efficient isolation of novel analogues and divergent total synthesis of jietacin A, B, C, and D.
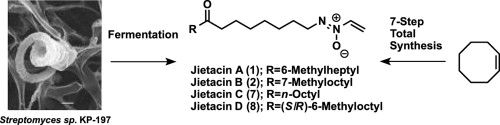
Akihiro Sugawara, Masahiko Kubo, Takuji Nakashima, Tomoyasu Hirose, Noriaki Tsunoda, Kyoichi Yahagi, Yukihiro Asami, Takeshi Yamada, Kazuro Shiomi, Yōko Takahashi, Satoshi Ōmura and Toshiaki Sunazuka
Tetrahedron 2015, 71, 2149-2157.
An architectonic macrolide library based on a C2-symmetric macrodiolide toward pharmaceutical compositions.
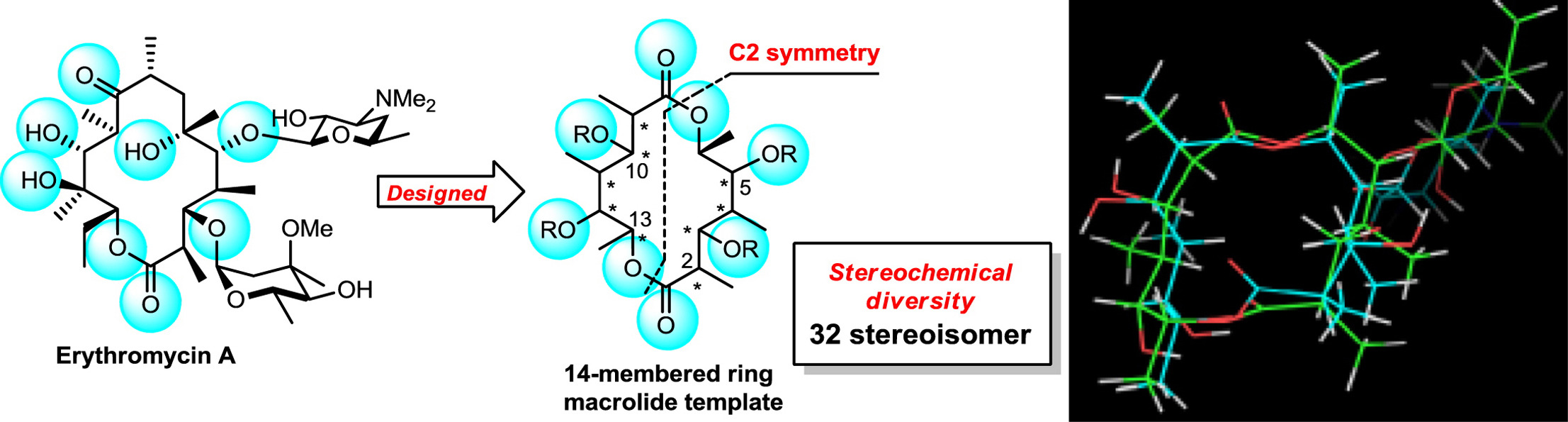
Invited article for Professor Jiro Tsuji special issue on the occasion of the 2014 Tetrahedron prize.
Hayato Nakano, Akihiro Sugawara, Tomoyasu Hirose, Hiroaki Gouda, Shuichi Hirono, Satoshi Ōmura and Toshiaki Sunazuka
Tetrahedron 2015, 71, 6569-6579.
Actinoallolides A–E, New Anti-trypanosomal Macrolides, Produced by an Endophytic Actinomycete, Actinoallomurus fulvus MK10-036.
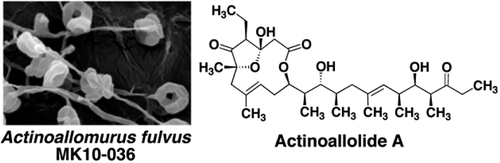
Yuki Inahashi, Masato Iwatsuki, Aki Ishiyama, Atsuko Matsumoto, Tomoyasu Hirose, Jun Oshita, Toshiaki Sunazuka, Watanalai Panbangred, Yoko Takahashi, Marcel Kaiser, Kazuhiko Otoguro and Satoshi Ōmura
Org. Lett. 2015, 17, 864-867.
Organocatalytic α-Addition of Isocyanides to Aldehydes.
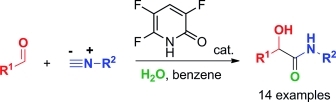
Takeshi Yamada, Tomoyasu Hirose, Satoshi Ōmura and Toshiaki Sunazuka
Eur. J. Org. Chem. 2015, 296–301.
2014
A concise total synthesis of puberulic acid, a potent antimalarial agent.
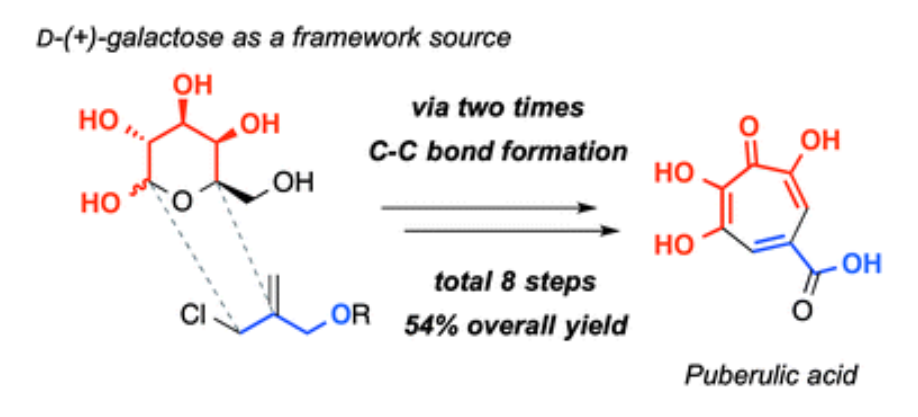
Goh Sennari, Tomoyasu Hirose, Masato Iwatsuki, Satoshi Ōmura and Toshiaki Sunazuka
Chem. Commun. 2014, 50, 8715-8718.
Leucomycin A3, a 16-membered macrolide antibiotic, inhibits influenza A virus infection and disease progression.
Ryuichi Sugamata, Akihiro Sugawara, Tomokazu Nagao, Koya Suzuki, Tomoyasu Hirose, Ki-ichi Yamamoto, Masamichi Oshima, Kazuo Kobayashi, Toshiaki Sunazuka, Kiyoko S Akagawa, Satoshi Ōmura, Toshinori Nakayama and Kazuo Suzuki
J. Antibiot. 2014, 67, 213-222.
2013
Observation of the controlled assembly of preclick components in the in situ click Chemistry generation of a chitinase inhibitor.
Tomoyasu Hirose, Nobuo Maita, Hiroaki Gouda, Jun Koseki, Tsuyoshi Yamamoto, Akihiro Sugawara, Hirofumi Nakano, Shuichi Hirono, Kazuro Shiomi, Takeshi Watanabe, Hisaaki Taniguchi, K. Barry Sharpless, Satoshi Ōmura and Toshiaki Sunazuka
Proc. Natl. Acad. Sci. USA, 2013, 110, 15892-15897.
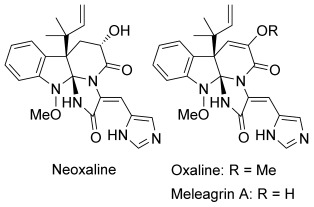
Asymmetric Total Synthesis of Neoxaline.
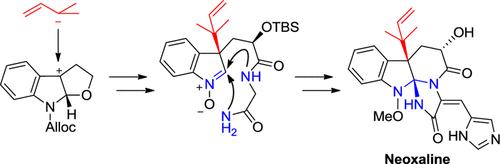
Tetsuya Ideguchi, Takeshi Yamada, Tatsuya Shirahata, Tomoyasu Hirose, Akihiro Sugawara, Yoshinori Kobayashi, Satoshi Ōmura and Toshiaki Sunazuka
J. Am. Chem. Soc. 2013, 135, 12568-12571.
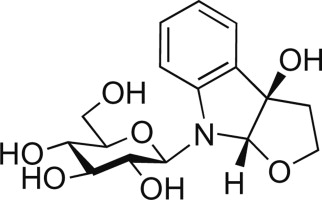
Structure Determination and Total Synthesis of (+)-16-Hydroxy-16,22-dihydroapparicine.
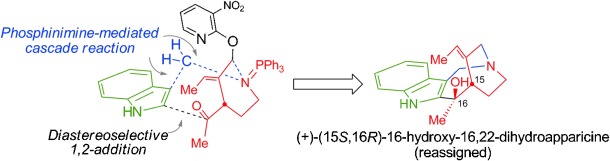
Tomoyasu Hirose, Yoshihiko Noguchi, Yujiro Furuya, Aki Ishiyama, Masato Iwatsuki, Kazuhiko Otoguro, Satoshi Ōmura and Toshiaki Sunazuka
Chem. Eur. J. 2013, 19, 10741-10750.
Human acidic mammalian chitinase as a novel target for anti-asthma drug design using in silico screening.
Masaki Wakasugi, Hiroaki Gouda, Tomoyasu Hirose, Akihiro Sugawara, Tsuyoshi Yamamoto, Kazuro Shiomi, Toshiaki Sunazuka, Satoshi Ōmura and Shuichi Hirono
Bioorg. Med. Chem. 2013, 21, 3214–3220.
Borrelidin analogues with antimalarial activity: design, synthesis and biological evaluation against Plasmodium falciparum parasites
Akihiro Sugawara, Toshiaki Tanaka, Tomoyasu Hirose, Aki Ishiyama, Yoko Takahashi, Kazuhiko Otoguro, Satoshi Ōmura and Toshiaki Sunazuka
Bioorg. Med. Chem. Lett. 2013, 23, 2302-2305.
EM, EM703 inhibit NF-kB activation induced by oxidative stress from diesel exhaust particle in human bronchial epithelial cells: Importance in IL-8 transcription.
YJ. Li, T. Shimizu, Y. Hirata, H. Inagaki, H. Takizawa, A. Azuma, T. Kawada, I. Sugawara, S. Kudoh, T. Sunazuka, and S. Ōmura
Pulm. Pharmacol. Ther. 2013, 26, 318-324.
Antimalarial C-9 oxime derivatives from desmycosin, produced by click chemistry.
Ayumi Tsutsui, Tomoyasu Hirose, Aki Ishiyama, Masato Iwatsuki, Arisa Yokota, Hitomi Maruyama, Hidetoshi Matsui, Kazuhiko Otoguro, Hideaki Hanaki, Satoshi Ōmura and Toshiaki Sunazuka
J. Antibiot. 2013, 66, 191-194.
2012
Wickerols A and B: novel anti-influenza virus diterpenes produced by Trichoderma atroviride FKI-3849.
Tsuyoshi Yamamoto, Naoyuki Izumi, Hideaki Ui, Akito Sueki, Rokuro Masuma, Kenichi Nonaka, Tomoyasu Hirose, Toshiaki Sunazuka, Takayuki Nagai, Haruki Yamada, Satoshi Ōmura and Kazuro Shiomi
Tetrahedron 2012, 68, 9267-9271.
Novel 12-membered non-antibiotic macrolides, EM900 series with anti-inflammatory and/or immunomodulatory activity; synthesis, structure–activity relationships and in vivo study.
Akihiro Sugawara, Akito Sueki, Tomoyasu Hirose, Hideaki Shima, Kiyoko S Akagawa, Satoshi Ōmura and Toshiaki Sunazuka
J. Antibiot. 2012, 65, 487-490.
Makomotindoline from Makomotake, Zizania latifolia infected with Ustilago esculenta.
Tomohiro Suzuki, Jae-Hoon Choi, Takumi Kawaguchi, Kimiko Yamashita, Akio Morita, Hirofumi Hirai, Kaoru Nagai, Tomoyasu Hirose, Satoshi Ōmura, Toshiaki Sunazuka and Hirokazu Kawagishi
Bioorg. Med. Chem. Lett. 2012, 22, 4246-4248.
マクロライドをはじめとする微生物由来生物活性天然物の合成研究と創薬展開.
Toward the total synthesis of Luminamicin: construction of 14-membered lactone framework possessing versatile enol ether moiety.
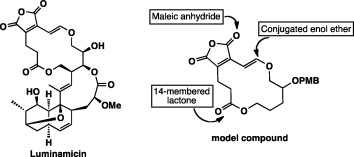
Aoi Kimishima, Tomoyasu Hirose, Akihiro Sugawara, Takanori Matsumaru, Kaoru Nakamura, Ken Katsuyama, Masaki Toda, Hirokazu Takada, Rokuro Masuma, Satoshi Ōmura, and Toshiaki Sunazuka
Tetrahedron Lett. 2012, 53, 2813-2816,
Three-dimensional solution structure of bottromycin A2: a potent antibiotic active against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococci.
Gouda H, Kobayashi Y, Yamada T, Ideguchi T, Sugawara A, Hirose T, Ōmura S, Sunazuka T, and Hirono S.
Chem. Pharm. Bull. 2012, 60, 169-171.
The first total synthesis and reassignment of the relative stereochemistry of 16-hydroxy-16,22-dihydroapparicine.
Y. Noguchi, Yoshihiko; Hirose, Tomoyasu; Furuya, Yujiro; Ishiyama, Aki; Otoguro, Kazuhiko; Ōmura, Satoshi and Sunazuka, ToshiakiY.
Tetrahedron Lett. 2012, 53, 1802-1807.
2011
Effects of a Novel Nonantibiotic Macrolide, EM900, on Cytokine and Mucin Gene Expression in a Human Airway Epithelial Cell Line
Otsu, K.; Ishinaga, H.; Suzuki, S.; Sugawara, A.; Sunazuka, T.; Ōmura, S.; Jono, H. and Takeuchi, K.
Pharmacology 2011, 88, 327–332.
Sequential one-pot glycosylation with glycosyl N-trichloroacetylcarbamate and trichloroacetate including dehydrative approach using 1-hydroxy sugars.
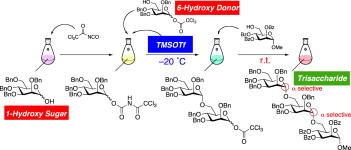
Tatsuya Shirahata, Asami Kojima, Satoko Teruya, Jun-ichi Matsuo, Masaki Yokoyama, Shogo Unagiike, Toshiaki Sunazuka, Kazuishi Makino, Eisuke Kaji, Satoshi Ōmura and Yoshinori Kobayashi
Tetrahedron 2011, 67, 6482-6496.
Solution-phase total synthesis of the hydrophilic natural product argifin using 3,4,5-tris(octadecyloxy)benzyl tag.
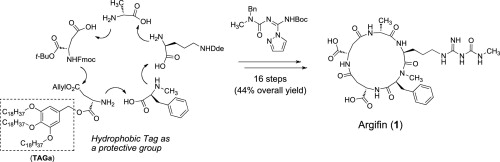
Tomoyasu Hirose, Takako Kasai, Takafumi Akimoto, Ayako Endo, Akihiro Sugawara, Kazuo Nagasawa, Kazuro Shiomi, Satoshi Ōmura and Toshiaki Sunazuka
Tetrahedron 2011, 67, 6633-6643.
Isolation, total synthesis and determination of the absolute configuration of Guadinomines; potent inhibitors of a bacterial type III secretion system.
Tomoyasu Hirose, Masato Iwatsuki, Satoshi, Ōmura, Toshiaki Sunazuka
Y. J. Soc. Org. Chem. Jpn. 2011, 69, 775-788.Y.
Novel 12-membered non-antibiotic macrolides from erythromycin A; EM900 series as novel leads for anti-inflammatory and/or immunomodulatory agents.
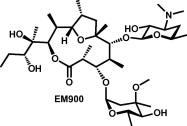
Akihiro Sugawara, Akito Sueki, Tomoyasu Hirose, Hideaki Shima, Kiyoko Akagawa, Satoshi Ōmura, Toshiaki Sunazuka
Bioorg. Med. Chem. Lett. 2011, 21, 3373-3376.
Spoxazomicins A-C, novel antitrypanosomal alkaloids produced by an endophytic actinomycete, Streptosporangium oxazolinicum K07-0460(T).
Inahashi Y. Iwatsuki M. Ishiyama A. Namatame M. Nishihara-Tsukashima A. Matsumoto A. Hirose T. Sunazuka T. Yamada H. Otoguro K. Takahashi Y. Ōmura S. and Shiomi K.
J. Antibiot. 2011, 64, 303-307.
Borrelidin, a potent antimalarial: stage-specific inhibition profile of synchronized cultures of Plasmodium falciparum.
Ishiyama A. Iwatsuki M. Namatame M. Nishihara-Tsukashima A. Sunazuka T. Takahashi Y. Ōmura S. and Otoguro K.
J. Antibiot. 2011, 64, 381-384.
2010
Bottromycin derivatives: efficient chemical modifications of the ester moiety and evaluation of anti-MRSA and anti-VRE activities.
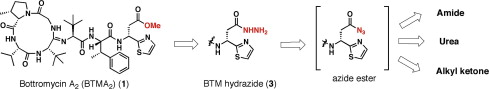
Yutaka Kobayashi, Maki Ichioka, Tomoyasu Hirose, Kenichiro Nagai, Atsuko Matsumoto, Hidehiro Matsui, Hideaki Hanaki, Rokuro Masuma, Yoko Takahashi, Satoshi Ōmura and Toshiaki Sunazuka
Bioorg. Med. Chem. Lett. 2010, 20, 6116-6120.
Boromycin derivatives: synthesis and antimalarial activity in vitro and in vivo.
Ayumi Tsutsui, Yujiro Furuya, Tomoyasu Hirose, Riyon Kim, Rokuro Masuma, Atsuko Matsumoto, Yoko Takahashi, Aki Ishiyama, Miyuki Namatame, Kazuhiko Otoguro, Satoshi Ōmura and Toshiaki Sunazuka
Heterocycles 2010, 82, 289-295. special issue dedicated to Professor Albert Eschenmoser
NMR spectroscopy and computational analysis of interaction between Serratia marcescens chitinase B and a dipeptide derived from natural-product cyclopentapeptide chitinase inhibitor argifin.
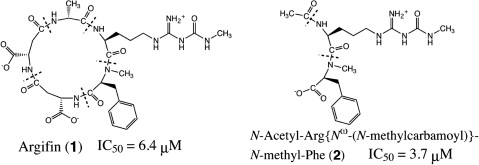
Gouda H.; Sunazuka T.; Hirose T.; Iguchi K.; Yamaotsu N.; Sugawara A.; Noguchi Y.; Saito Y.; Yamamoto T.; Watanabe T.; Shiomi K.; Ōmura S. and Hirono S.
Bioorg. Med. Chem. 2010, 18, 5835-5844.
Oral adjuvant activity for nasal influenza vaccines caused by combination of two trihydroxy fatty acid stereoisomers from the tuber of Pinellia ternata.
Nagai T.; Shimizu Y.; Shirahata T.; Sunazuka T.; Kiyohara H;. Ōmura S. and Yamada H.
Int Immunopharmacol. 2010, 10, 655-661.
Improved catalytic and stereoselective glycosylation with glycosyl N-trichloroacetylcarbamate: application to various 1-hydroxy sugars.
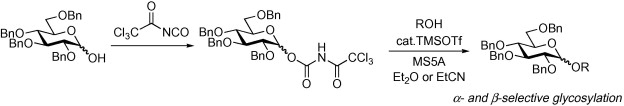
Shirahata T.; Matsuo J.; Teruya S.; Hirata N.; Kurimoto T.; Akimoto N.; Sunazuka T.; Kaji E. and Ōmura S.
Carbohydr. Res. 2010, 345, 740-749.
Recent development of two chitinase inhibitor, Argifin and Argadin, produced by soil microorganisms
Tomoyasu Hirose, Toshiaki Sunazuka and Satoshi Ōmura
Proc. Jpn. Acad., Ser. B, 2010, 86, 85-102.
Effect of erythromycin A and its new derivative EM201 on type I collagen production by cultured dermal fibroblasts.
Namikawa H., Sunazuka T., Kitamura Y., Suzuki T., Hamasaki Y., Yamazaki S., Ōmura S., Hatamochi A.
Y. Arch Dermatol Res. 2010, 302, 341-348.Y.
2009
The selectivity of beauveriolide derivatives in inhibition toward the two isozymes of acyl-CoA: cholesterol acyltransferase.
Ohshiro T., Matsuda D., Nagai K., Doi T., Sunazuka T., Takahashi T., Rudel L. L., Ōmura S. and Tomoda H.
Chem. Pharm. Bull. 2009, 57, 377-381.
Solid-phase synthesis and biological activity of malformin C and its derivatives
Yasuhiro Kojima , Toshiaki Sunazuka, Kenichiro Nagai, Tomoyasu Hirose, Miyuki Namatame , Aki Ishiyama , Kazuhiko Otoguro and Satoshi Ōmura
J. Antibiot. 2009, 62, 681-686.
Molecular modeling of human acidic mammalian chitinase in complex with the natural-product cyclopentapeptide chitinase inhibitor argifin.
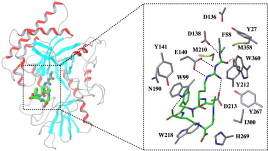
Hiroaki Gouda, Shinichi Terashima, Kanami Iguchi, Akihiro Sugawara, Yoshifumi Saito, Tsuyoshi Yamamoto, Tomoyasu Hirose, Kazuro Shiomi, Toshiaki Sunazuka, Satoshi Ōmura and Shuichi Hirono
Bioorg. Med. Chem. 2009, 17, 6270-6278.
Solid-phase total synthesis of the chitinase inhibitor Argadin using a supported acetal resin.
Tomoyasu Hirose, Toshiaki Sunazuka, Akihiro Sugawara, Yoshihiko Noguchi, Toshiaki Tanaka, Kanami Iguchi, Tsuyoshi Yamamoto, Hiroaki Gouda, Kazuro Shiomi and Satoshi Ōmura
J. Antibiot. 2009, 62, 495-500.
Role of Dexamethasone and Oncostatin M on the Formation of Vacuoles in Human Fetal Liver Cells.
Tsuyoshi Teramoto, Tamihide Matsunaga, Mie Toba, Toshiaki Sunazuka, Satoshi Ōmura and Shigeru Ohmori
Biol. Pharm. Bull. 2009, 32, 209-212.
Chitinase inhibitors: extraction of the active framework from natural argifin and use of in situ click chemistry.
Tomoyasu Hirose, Toshiaki Sunazuka, Akihiro Sugawara, Ayako Endo, Kanami Iguchi, Tsuyoshi Yamamoto, Hideaki Ui, Kazuro Shiomi, Takeshi Watanabe, K. Barry Sharpless and Satoshi Ōmura
J. Antibiot. 2009, 62, 277-282.
Computer-aided rational molecular design of argifin-derivatives with more potent inhibitory activity against chitinase B from Serratia marcescens.
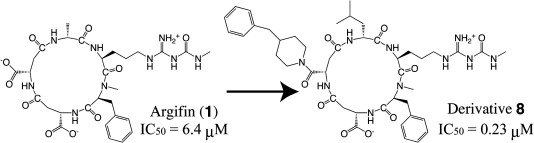
Hiroaki Gouda, Toshiaki Sunazuka, Kanami Iguchi, Akihiro Sugawara, Tomoyasu Hirose, Yoshihiko Noguchi, Yoshifumi Saito, Yuichi Yanai, Tsuyoshi Yamamoto, Takeshi Watanabe, Kazuro Shiomi,Satoshi Ōmura and Shuichi Hirono
Bioorg. Med. Chem. Lett. 2009, 17, 2630-2633.
Argifin; Efficient Solid Phase Total Synthesis and Unexpected Discovery of potent Acyclic peptides.
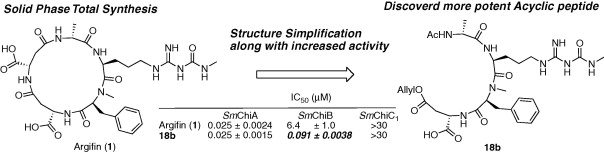
Toshiaki Sunazuka, Akihiro Sugawara, Kanami Iguchi, Tomoyasu Hirose, Kenichiro Nagai, Yoshihiko Noguchi, Yoshihumi Saito, Hiroaki Gouda, Tsuyoshi Yamamoto, Hideaki Ui, Kazuro Shiomi and Satoshi Ōmura
Bioorg. Med. Chem. 2009, 17, 2751-2758.
Structure Determination and Total Synthesis of Bottromycin A2: A Potent Antibiotic against MRSA and VRE.
Shimamura, Hiroyuki; Gouda, Hiroaki; Nagai, Kenichiro; Hirose, Tomoyasu; Ichioka, Maki; Furuya, Yujiro; Kobayashi, Yutaka; Hirono, Shuichi; Sunazuka, Toshiaki and Ōmura, Satoshi
Angew. Chem. Int. Ed. 2009, 48, 914-917.
2008
Total Synthesis and Determination of the Absolute Configuration of Guadinomines B and C2.
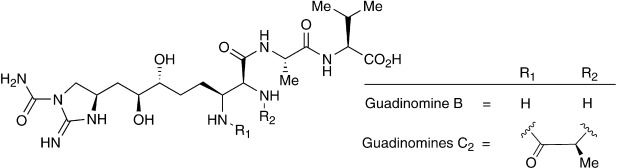
Hirose, Tomoyasu; Sunazuka, Toshiaki; Tsuchiya, Satoshi; Tanaka, Toshiaki; Kojima, Yasuhiro; Iwatsuki, Masato and Ōmura, Satoshi
Chem. Eur. J. 2008, 14, 8220-8238.
Selectivity of pyripyropene derivatives in inhibition toward acyl-CoA:cholesterol acyltransferase 2 isozyme.
Ohshiro, Taichi; Ohte, Satoshi; Matsuda, Daisuke; Ohtawa, Masaki; Nagamitsu, Tohru; Sunazuka, Toshiaki; Harigaya, Yoshihiro; Rudel, Lawrence L.; Ōmura, Satoshi and Tomoda, Hiroshi
J. Antibiot. 2008, 61, 503-508.
Erythromycin derivatives inhibit HIV-1 replication in macrophages through modulation of MAPK activity to induce small isoforms of C/EBPβ.
Komuro, Iwao; Sunazuka, Toshiaki; Akagawa, Kiyoko S.; Yokota, Yasuko; Iwamoto, Aikichi and Ōmura, Satoshi
Proc. Nat. Acad. Sci. USA. 2008, 105, 12509-12514.
Synthesis and biological evaluation of a focused library of beauveriolides.
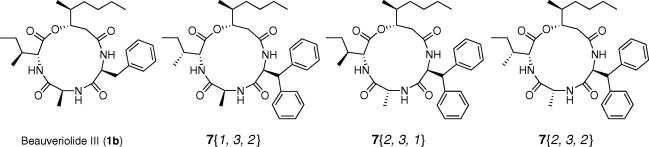
Nagai, Kenichiro; Doi, Takayuki; Ohshiro, Taichi; Sunazuka, Toshiaki; Tomoda, Hiroshi; Takahashi, Takashi and Ōmura, Satoshi
Bioorg. Med. Chem. Lett. 2008, 18, 4397-4400.
EM703, A NEW DERIVATIVE OF ERYTHROMYCIN, INHIBITS TRANSFORMING GRWTH FACTOR-β SIGNALING IN HUMAN LUNG FIBROBLASTS.
Yu, ChangHe; Azuma, Arata; Li, YingJi; Wang, Chunyan; Abe, Sinji; Usuki, Jiro; Matsuda, Kuniko; Kudoh, Shoji; Sunazuka, Toshiaki and Ōmura, Satoshi
Experimental Lung Research 2008, 34, 343-354.
Total synthesis of malformin C, an inhibitor of bleomycin-induced G2 arrest.
Kojima, Yasuhiro; Sunazuka, Toshiaki; Nagai, Kenichiro; Julfakyan, Khachatur; Fukuda, Takashi; Tomoda, Hiroshi and Ōmura, Satoshi
J. Antibiot. 2008, 61, 297-302.
Synthesis and biological properties of tensyuic acids B, C, and E, and investigation of the optical purity of natural tensyuic acid B.
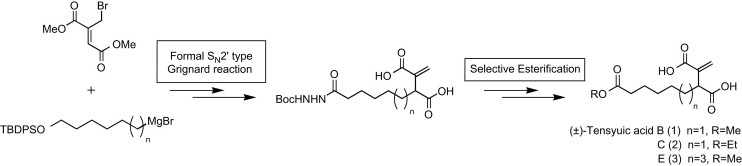
Matsumaru, Takanori; Sunazuka, Toshiaki; Hirose, Tomoyasu; Ishiyama, Aki; Namatame, Miyuki; Fukuda, Takashi; Tomoda, Hiroshi; Otoguro, Kazuhiko and Ōmura, Satoshi
Tetrahedron 2008, 64, 7369-7377.
Guadinomines, Type III secretion system inhibitors, produced by Streptomyces sp. K01-0509. II. Physico-chemical properties and structure elucidation.
Iwatsuki, Masato; Uchida, Ryuji; Yoshijima, Hitomi; Ui, Hideaki; Shiomi, Kazuro; Kim, Yong-Pil; Hirose, Tomoyasu; Sunazuka, Toshiaki; Abe, Akio; Tomoda, Hiroshi and Ōmura, Satoshi
J. Antibiot. 2008, 61, 230-236.
4”’-N-demethylspiramycin derivatives: Synthesis and evaluation of effectiveness against drug-resistant bacteria.
Sunazuka, Toshiaki; Shudo, Hiroko; Nagai, Kenichiro; Yoshida, Kiminari; Yamaguchi, Yukie; Hanaki, Hideaki and Ōmura, Satoshi
J. Antibiot. 2008, 61, 175-184.
Computational analysis of the binding affinities of the natural-product cyclopentapeptides argifin and argadin to chitinase B from Serratia marcescens.
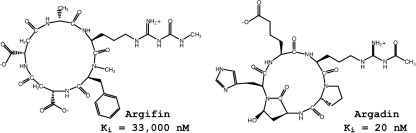
Gouda, Hiroaki; Yanai, Yuichi; Sugawara, Akihiro; Sunazuka, Toshiaki; Ōmura, Satoshi and Hirono, Shuichi
Bioorg. Med. Chem. 2008, 16, 3565-3579.
EM703, the new derivative of erythromycin, inhibits transcription of type I collagen in normal and scleroderma fibroblasts.
Ikeda, Hideyuki; Sunazuka, Toshiaki; Suzuki, Hiromi; Hamasaki, Yoichiro; Yamazaki, Soji; Ōmura, Satoshi and Hatamochi, Atsushi
Journal of Dermatological Science 2008, 49, 195-205.
Efficient Total Synthesis of Novel Bioactive Microbial Metabolites.
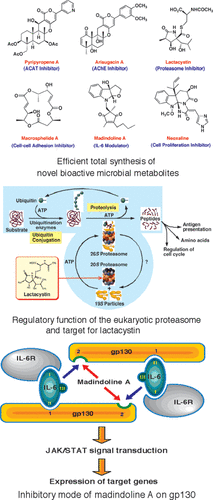
Sunazuka, Toshiaki; Hirose, Tomoyasu and Ōmura, Satoshi
Acc. Chem. Res. 2008, 41, 302-314.
2007
A novel natural compound, cycloanthranilylproline derivative (Fuligocandin B), sensitizes leukemia cells to apoptosis induced by tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) through 15-deoxy- 12,14 prostaglandinJ2 production.
Hiroo Hasegawa, Yasuaki Yamada,Kanki Komiyama, Masahiko Hayashi, Masami Ishibashi, Toshiaki Sunazuka, Takeshi Izuhara, Kazuyuki Sugahara, Kazuto Tsuruda, Masato Masuda, Nobuyuki Takasu, Kunihiro Tsukasaki, Masao Tomonaga and Shimeru Kamihira
Blood 2007, 110, 1664-1674.
Design and synthesis via click chemistry of 8,9-anhydroerythromycin A 6,9-hemiketal analogues with anti-MRSA and -VRE activity.
Sugawara, Akihiro; Sunazuka, Toshiaki; Hirose, Tomoyasu; Nagai, Kenichiro; Yamaguchi, Yukie; Hanaki, Hideaki; Sharpless, K. Barry and Ōmura, Satoshi
Bioorg. Med. Chem. Lett. 2007, 17, 6340-6344.
Synthesis of the oxa-bridged octalin system of two anti-anaerobe antibiotics, luminamicin and lustromycin.
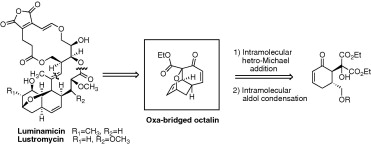
Sunazuka, Toshiaki; Handa, Masaki; Hirose, Tomoyasu; Matsumaru, Takanori; Togashi, Yuko; Nakamura, Kaoru; Iwai, Yuzuru and Ōmura, Satoshi
Tetrahedron Lett. 2007, 48, 5297-5300.
A new method for efficient coupling of indole and epoxide catalyzed with Yb(OTF)3, and application to the total synthesis of kurasoin B.
Tsuchiya, Satoshi; Sunazuka, Toshiaki; Shirahata, Tatsuya; Hirose, Tomoyasu; Kaji, Eisuke and Ōmura, Satoshi
Heterocycles 2007, 72, 91-94.
Studies toward the total synthesis of irumamycin: stereoselective preparation of the C(15)-C(27) segment via two-directional chain synthesis.
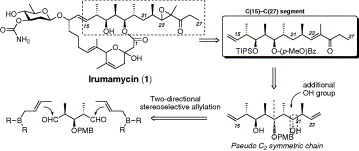
Hirose, Tomoyasu; Sunazuka, Toshiaki; Yamamoto, Daisuke; Mouri, Mutsumi; Hagiwara, Yoshiaki; Matsumaru, Takanori; Kaji, Eisuke and Ōmura, Satoshi
Tetrahedron Lett. 2007, 48, 413-416.
Total Synthesis and Biological Evaluation of Verticipyrone and Analogues.
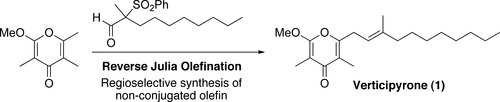
Shimamura, Hiroyuki; Sunazuka, Toshiaki; Izuhara, Takashi; Hirose, Tomoyasu; Shiomi, Kazuro and Ōmura, Satoshi
Org. Lett. 2007, 9, 65-67.
2006
EM703 Improves Bleomycin-induced Pulmonary Fibrosis in Mice by The Inhibition of TGF-β Signaling in Lung Fibroblasts.
Li, Y. J., Azuma, A., Usuki, J., Abe, S., Matsuda, K., Sunazuka, T., Shimizu, T., Hirata, Y., Inagaki, H., Kawada, T., Takahashi, S., Kudoh, S. and Omura, S.
Respiratory Research 2006, 7, 16-28.
Verticipyrone, a new NADH-fumarate reductase inhibitor, produced by Verticillium sp. FKI-1083.
Ui, Hideaki; Shiomi, Kazuro; Suzuki, Hideaki; Hatano, Hiroko; Morimoto, Hiromi; Yamaguchi, Yuichi; Masuma, Rokuro; Sunazuka, Toshiaki; Shimamura, Hiroyuki; Sakamoto, Kimitoshi; Kita, Kiyoshi; Miyoshi, Hideto; Tomoda, Hiroshi and Ōmura, Satoshi
J. Antibiot. 2006, 59, 785-790.
Rapid ‘SAR’ via click chemistry: an alkyne-bearing spiramycin is fused with diverse azides to yield new triazole-antibacterial candidates.
Hirose, Tomoyasu; Sunazuka, Toshiaki; Noguchi, Yoshihiko; Yamaguchi, Yukie; Hanaki, Hideaki; Sharpless, K. Barry and Ōmura, Satoshi
Heterocycles 2006, 69, 55-61.
Asymmetric Total Synthesis of (+)-K01-0509 B: Determination of Absolute Configuration.
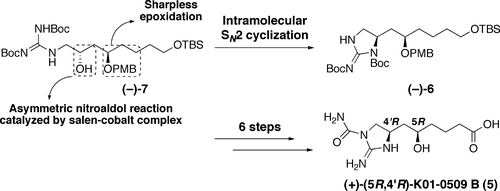
Tsuchiya, Satoshi; Sunazuka, Toshiaki; Hirose, Tomoyasu; Mori, Ryuma; Tanaka, Toshiaki; Iwatsuki, Masato and Ōmura, Satoshi
Org. Lett. 2006, 8, 5577-5580.
Verticilide: Elucidation of Absolute Configuration and Total Synthesis.
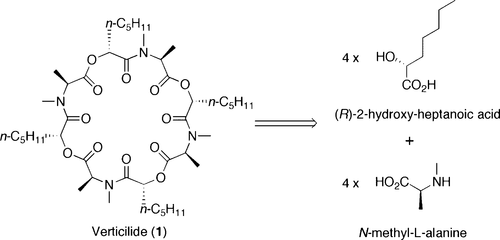
Monma, Souichi; Sunazuka, Toshiaki; Nagai, Kenichiro; Arai, Takahiro; Shiomi, Kazuro; Matsui, Ryosuke and Ōmura, Satoshi
Org. Lett. 2006, 8, 5601-5604.
Total synthesis, elucidation of absolute stereochemistry, and adjuvant activity of trihydroxy fatty acids.
Shirahata, Tatsuya; Sunazuka, Toshiaki; Yoshida, Kiminari; Yamamoto, Daisuke; Harigaya, Yoshihiro; Kuwajima, Isao; Nagai, Takayuki; Kiyohara, Hiroaki; Yamada, Haruki and Ōmura, Satoshi
Tetrahedron 2006, 62, 9483-9496.
Synthetic applications of a three-component Mannich reaction. Total synthesis of IL-6 inhibitor (+)-madindoline A and B.
Hirose, Tomoyasu; Sunazuka, Toshiaki; Yamamoto, Daisuke; Kaji, Eisuke and Ōmura, Satoshi
Tetrahedron Lett. 2006, 47, 6761-6764.
Synthesis and biological evaluation of a beauveriolide analogue library.
Doi, Takayuki; Nagai, Kenichiro; Sekiguchi, Takafumi; Fujimoto, Nobuaki; Namatame, Ichiji; Sunazuka, Toshiaki; Tomoda, Hiroshi; Ōmura, Satoshi and Takahashi, Takashi
Peptide Science 2006, 42, 51-52.
Design, synthesis, and biological activities of madindoline analogs.
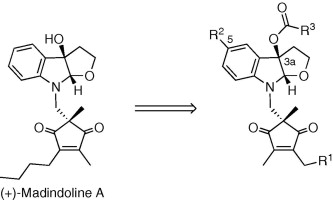
Yamamoto, Daisuke; Sunazuka, Toshiaki; Hirose, Tomoyasu; Kojima, Naoto; Kaji, Eisuke and Ōmura, Satoshi
Bioorg. Med. Chem. Lett. 2006, 16, 2807-2811.
Three-dimensional solution structure of EM703 with potent promoting activity of monocyte-to-macrophage differentiation.
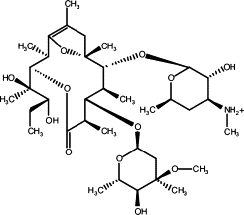
Gouda, Hiroaki; Sunazuka, Toshiaki; Yoshida, Kiminari; Sugawara, Akihiro; Sakoh, Yusuke; Ōmura, Satoshi and Hirono, Shuichi.
Bioorg. Med. Chem. Lett. 2006, 16, 2496-2499.
Synthesis and Biological Evaluation of a Beauveriolide Analogue Library.
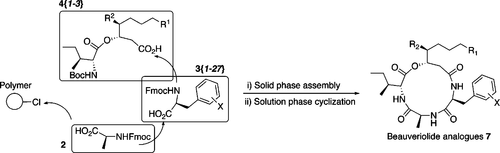
Nagai, Kenichiro; Doi, Takayuki; Sekiguchi, Takafumi; Namatame, Ichiji; Sunazuka, Toshiaki; Tomoda, Hiroshi; Ōmura, Satoshi and Takahashi, Takashi.
J. Comb. Chem. 2006, 8, 103-109.
2005
Stereostructure of luminamicin, an anaerobic antibiotic, via molecular dynamics, NMR spectroscopy, and the modified Mosher method.
Gouda, Hiroaki; Sunazuka, Toshiaki; Ui, Hideaki; Handa, Masaki; Sakoh, Yusuke; Iwai, Yuzuru; Hirono, Shuichi and Ōmura, Satoshi
Proc. Nat. Acad. Sci. USA. 2005, 102, 18286-18291.
Total Synthesis of α-Pyrone Meroterpenoids, Novel Bioactive Microbial Metabolites.
Total synthesis of madindolines, potent selective inhibitors of interleukine 6, novel bioactive microbial metabolites.
Sunazuka, Toshiaki; Hirose, Tomoyasu and Ōmura, Satoshi
Yuki Gosei Kagaku Kyokaishi, 2005, 63, 1090-1101.
Azithromycin inhibits the formation of flagellar filaments without suppressing flagellin synthesis in Salmonella enterica serovar Typhimurium.
Matsui, Hidenori; Eguchi, Masahiro; Ohsumi, Katsufumi; Nakamura, Akio; Isshiki, Yasunori; Sekiya, Kachiko; Kikuchi, Yuji; Nagamitsu, Tohru; Masuma, Rokuro; Sunazuka, Toshiaki and Ōmura, Satoshi
Antimicrob. Agents Chemother. 2005, 49, 3396-3403.
Determination of the absolute stereochemistry and asymmetric total synthesis of madindolines A and B: a practical improvement to a second-generation approach from the first-generation.
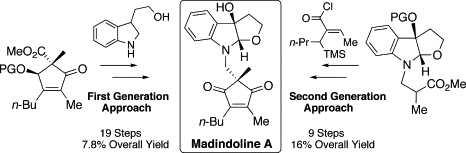
Hirose, Tomoyasu; Sunazuka, Toshiaki; Yamamoto, Daisuke; Kojima, Naoto; Shirahata, Tatsuya; Harigaya, Yoshihiro; Kuwajima, Isao and Ōmura, Satoshi
Tetrahedron 2005, 61, 6015-6039.
Absolute stereochemistries and total synthesis of (+)/(-)-macrosphelides, potent, orally bioavailable inhibitors of cell-cell adhesion.
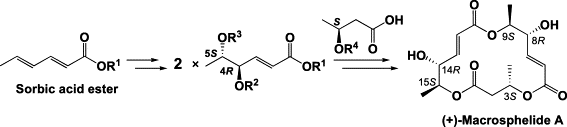
Sunazuka, Toshiaki; Hirose, Tomoyasu; Chikaraishi, Noriko; Harigaya, Yoshihiro; Hayashi, Masahiko; Komiyama, Kanki; Sprengeler, Paul A.; Smith, Amos B. and Ōmura, Satoshi
Tetrahedron 2005, 61, 3789-3803.
A Concise Stereoselective Route to the Indoline Spiroaminal Framework of Neoxaline and Oxaline.
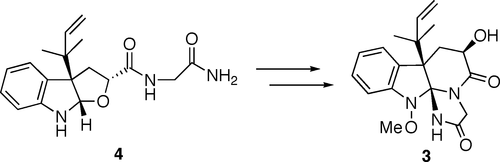
Sunazuka, Toshiaki; Shirahata, Tatsuya; Tsuchiya, Satoshi; Hirose, Tomoyasu; Mori, Ryuma; Harigaya, Yoshihiro; Kuwajima, Isao and Ōmura, Satoshi
Org. Lett. 2005, 7, 941-943.
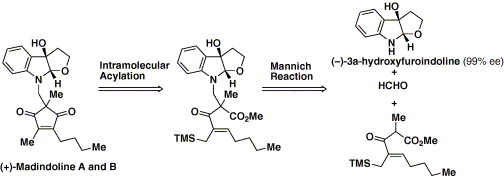
Total synthesis of (-)-physovenine from (-)-3a-hydroxyfuroindoline.
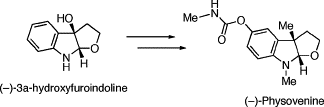
Sunazuka, Toshiaki; Yoshida, Kiminari; Kojima, Naoto; Shirahata, Tatsuya; Hirose, Tomoyasu; Handa, Masaki; Yamamoto, Daisuke; Harigaya, Yoshihiro; Kuwajima, Isao and Ōmura, Satoshi
etrahedron Lett. 2005, 46, 1459-1461.
Macrolides with promotive activity of monocyte to macrophage differentiation
Yoshida, Kiminari; Sunazuka, Toshiaki; Nagai, Kenichiro; Sugawara, Akihiro; Cho, Achim; Nagamitsu, Tohru; Harigaya, Yoshihiro; Otoguro, Kazuhiko; Akagawa, Kiyoko S. and Ōmura, Satoshi
J. Antibiot. 2005, 58, 79-81.
2004
Absolute stereochemistries and total synthesis of (+)-arisugacins A and B, potent, orally bioactive and selective inhibitors of acetylcholinesterase.
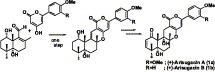
Sunazuka, Toshiaki; Handa, Masaki; Nagai, Kenichiro; Shirahata, Tatsuya; Harigaya, Yoshihiro; Otoguro, Kazuhiko; Kuwajima, Isao and Ōmura, Satoshi
Tetrahedron 2004, 60, 7845-7859.
Synthesis and biological evaluation of novel 4”-alkoxy avermectin derivatives.
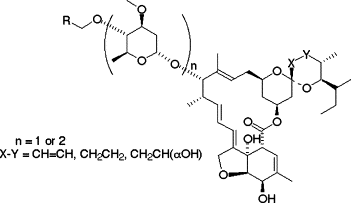
Nagai, Kenichiro; Shiomi, Kazuro; Sunazuka, Toshiaki; Harder, Achim; Turberg, Andreas and Ōmura, Satoshi
Bioorg. Med. Chem. Lett. 2004, 14, 4135-4139.
In vitro and in vivo antimalarial activities of a carbohydrate antibiotic, prumycin, against drug-resistant strains of Plasmodium.
Otoguro, Kazuhiko; Ishiyama, Aki; Kobayashi, Miyuki; Sekiguchi, Hitomi; Izuhara, Takashi; Sunazuka, Toshiaki; Tomoda, Hiroshi; Yamada, Haruki and Ōmura, Satoshi
J. Antibiot. 2004, 57, 400-402.
Molecular mechanisms of anti-inflammatory action of erythromycin in human bronchial epithelial cells: Possible role in the signaling pathway that regulates nuclear factor-κ B activation.
Desaki, Masashi; Okazaki, Hitoshi; Sunazuka, Toshiaki; Ōmura, Satoshi; Yamamoto, Kazuhiko and Takizawa, Hajime
Antimicrob. Agents Chemother. 2004, 48, 1581-1585.
Synthesis of 4”-alkoxy avermectin derivatives using rhodium carbenoid-mediated O-H insertion reaction.

Nagai, Kenichiro; Sunazuka, Toshiaki and Ōmura, Satoshi
Tetrahedron Lett. 2004, 45, 2507-2509.
2003
A combinatorial synthesis of a macrosphelide library utilizing a palladium-catalyzed carbonylation on a polymer support.
Takahashi, Takashi; Kusaka, Shin-ichi; Doi, Takayuki; Sunazuka, Toshiaki and Ōmura, Satoshi.
Angew. Chem. Int. Ed. 2003, 42, 5230-5234.
Synthesis and biological activities of novel 4”-alkylidene avermectin derivatives.
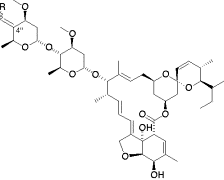
Nagai, Kenichiro; Sunazuka, Toshiaki; Shiomi, Kazuro; Harder, Achim; Turberg, Andreas and Ōmura, Satoshi
Bioorg. Med. Chem. Lett. 2003, 13, 3943-3946.
Total syntheses of the AChE inhibitors (±)-arisugacins F and G.
Handa, Masaki; Sunazuka, Toshiaki; Sugawara, Akihiro; Harigaya, Yoshihiro; Otoguro, Kazuhiko and Ōmura, Satoshi
J. Antibiot. 2003, 56, 730-733.
Effect of 14-membered macrolide compounds on monocyte to macrophage differentiation.
Sunazuka, Toshiaki; Yoshida, Kiminari; Oohori, Masako; Otoguro, Kazuhiko; Harigaya, Yoshihiro; Iwai, Yuzuru; Akagawa, Kiyoko S. and Ōmura, Satoshi
J. Antibiot. 2003, 56, 721-724.
Structure determination of lustromycin, an antibiotic against anaerobic bacteria.
Handa, Masaki; Ui, Hideaki; Yamamoto, Daisuke; Monma, Soichi; Iwai, Yuzuru; Sunazuka, Toshiaki and Ōmura, Satoshi
Heterocycles 2003, 59, 497-500.
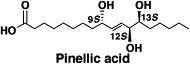
Total synthesis and adjuvant activity of all stereoisomers of pinellic acid.
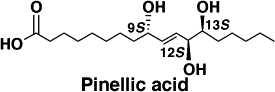
Shirahata, Tatsuya; Sunazuka, Toshiaki; Yoshida, Kiminari; Yamamoto, Daisuke; Harigaya, Yoshihiko; Nagai, Takayuki; Kiyohara, Hiroaki; Yamada, Haruki; Kuwajima, Isao and Ōmura, Satoshi
Bioorg. Med. Chem. Lett. 2003, 13, 937-941.
2002
Suppression of bone resorption by madindoline A, a novel nonpeptide antagonist to gp130.
Hayashi, Masahiko; Rho, Mun-Chual; Enomoto, Akiko; Fukami, Akiko; Kim, Yong-Pil; Kikuchi, Yuji; Sunazuka, Toshiaki; Hirose, Tomoyasu; Komiyama, Kanki and Ōmura, Satoshi
Proc. Nat. Acad. Sci. USA. 2002, 99, 14728-14733.
Pinellic acid from the tuber of Pinellia ternata Breitenbach as an effective oral adjuvant for nasal influenza vaccine.
Nagai, Takayuki; Kiyohara, Hiroaki; Munakata, Kaori; Shirahata, Tatsuya; Sunazuka, Toshiaki; Harigaya, Yoshihiro and Yamada, Haruki
International Immunopharmacology 2002, 2, 1183-1193.
Fourteen-member macrolides suppress interleukin-8 production but do not promote apoptosis of activated neutrophils.
Tsuchihashi, Yoshiko; Oishi, Kazunori; Yoshimine, Hiroyuki; Suzuki, Shoichi; Kumatori, Atsushi; Sunazuka, Toshiaki; Ōmura, Satoshi; Matsushima, Kouji and Nagatake, Tsuyoshi
Antimicrob. Agents Chemother. 2002, 46, 1101-1104.
Total synthesis of pinellic acid, a potent oral adjuvant for nasal influenza vaccine. Determination of the relative and absolute configuration.
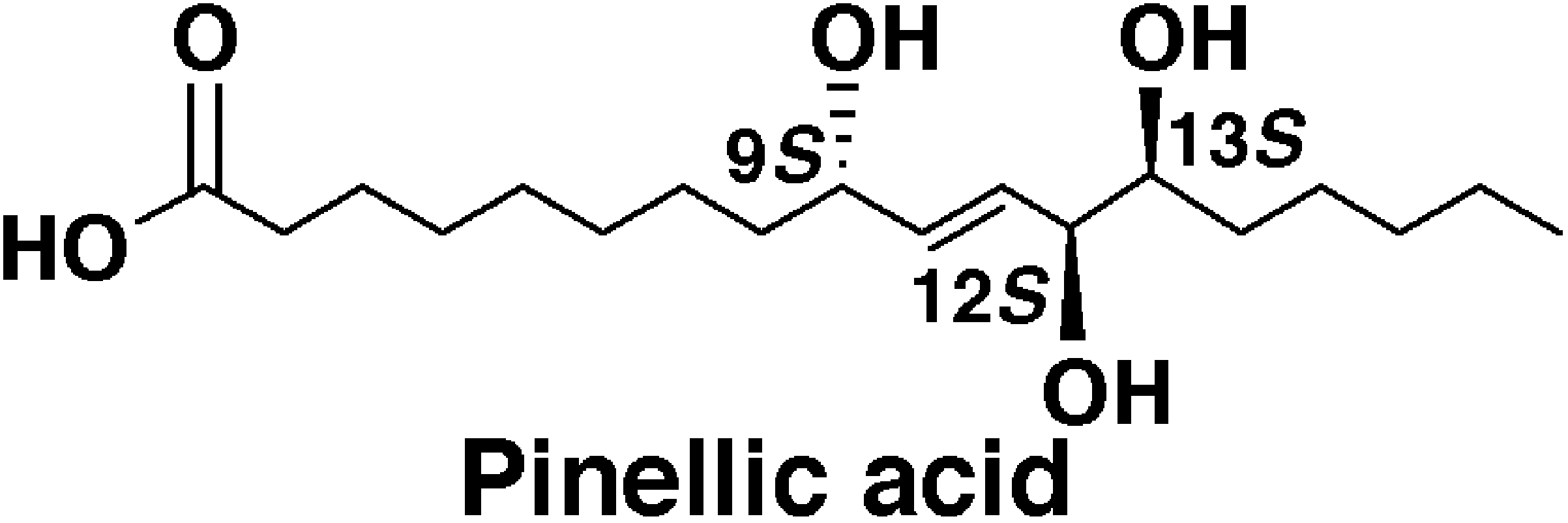
Sunazuka, Toshiaki; Shirahata, Tatsuya; Yoshida, Kiminari; Yamamoto, Daisuke; Harigaya, Yoshihiro; Nagai, Takayuki; Kiyohara, Hiroaki; Yamada, Haruki; Kuwajima, Isao and Ōmura, Satoshi
Tetrahedron Lett. 2002, 43, 1265-1268.
Short Total Synthesis of (+)-Madindolines A and B.
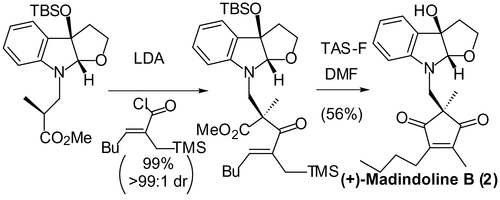
Hirose, Tomoyasu; Sunazuka, Toshiaki; Shirahata, Tatsuya; Yamamoto, Daisuke; Harigaya, Yoshihiro; Kuwajima, Isao and Ōmura, Satoshi
Org. Lett. 2002, 4, 501-503.
The First Total Synthesis of (±)-Arisugacin A, a Potent, Orally Bioavailable Inhibitor of Acetylcholinesterase.
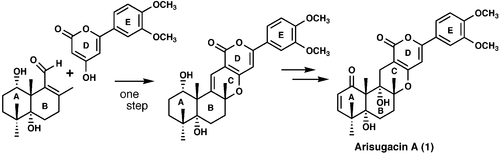
Sunazuka, Toshiaki; Handa, Masaki; Nagai, Kenichiro; Shirahata, Tatsuya; Harigaya, Yoshihiro; Otoguro, Kazuhiko; Kuwajima, Isao and Ōmura, Satoshi
Org. Lett. 2002, 4, 367-369.